As a researcher in the field of energy storage, I have observed the growing interest in all-solid-state batteries due to their potential to overcome the limitations of conventional liquid lithium-ion batteries. By replacing flammable organic electrolytes with solid-state electrolytes, these batteries offer enhanced safety and the possibility of higher energy densities when paired with advanced electrode materials. However, the unique characteristics of solid-state batteries, such as their ion transport mechanisms, interface stability, thermal behavior, and environmental adaptability, necessitate the development of a comprehensive testing and evaluation system. In this article, I will discuss the construction of such a system across multiple levels—from materials and interfaces to single cells, systems, and vehicles—and highlight the challenges and future directions for testing technologies.
Solid-state batteries differ fundamentally from liquid-based systems in several aspects. For instance, ion transport in solid-state batteries relies on physical contact between solid particles, often resulting in “point contact” interfaces that limit ion conduction efficiency. This can be described by the following equation for ionic conductivity: $$\sigma = \frac{L}{R \cdot A}$$ where $\sigma$ is the ionic conductivity, $L$ is the thickness of the electrolyte, $R$ is the resistance, and $A$ is the contact area. In contrast, liquid electrolytes provide uniform wetting, ensuring consistent ion pathways. Additionally, the absence of liquid components in solid-state batteries reduces risks like leakage and thermal runaway, but introduces new challenges in maintaining interface integrity and managing mechanical stress.
To address these differences, a multi-level testing framework is essential. Below, I outline the key components of this framework, incorporating tables and formulas to summarize critical aspects.
New Characteristics of Solid-State Batteries and Testing Focus
Solid-state batteries exhibit distinct features that redefine testing priorities. For example, ion transport efficiency is highly dependent on particle size and pressure, as smaller particles and higher pressures can enhance contact areas. The relationship between pressure and conductivity can be modeled as: $$\sigma(P) = \sigma_0 \cdot e^{kP}$$ where $\sigma_0$ is the baseline conductivity, $P$ is the applied pressure, and $k$ is a constant related to material properties. Interface stability is another critical area; unlike liquid systems where interfaces are self-healing, solid-solid interfaces in solid-state batteries are prone to degradation over cycles due to volume changes and chemical reactions. Thermal safety is improved, with onset temperatures for thermal runaway often exceeding 200°C, compared to 130–200°C in liquid batteries. However, the concentrated energy release in solid-state batteries during failure can be more severe, requiring advanced safety evaluations.
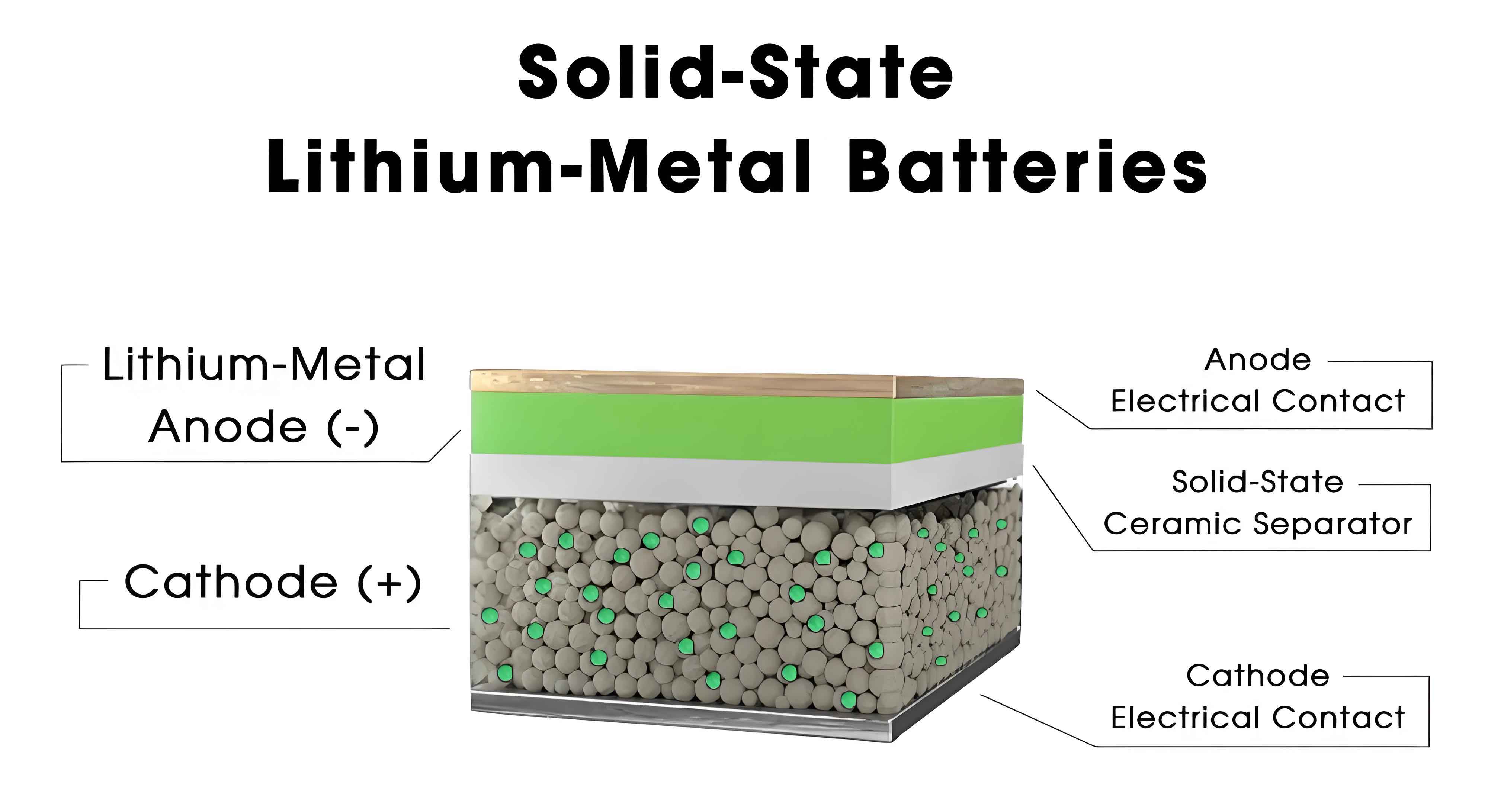
Multi-Level Testing and Evaluation System
Material-Level Testing
At the material level, solid-state electrolytes must be evaluated for ionic conductivity, electronic conductivity, electrochemical stability, mechanical properties, and thermal characteristics. For instance, ionic conductivity is typically measured using electrochemical impedance spectroscopy (EIS), and the results can be summarized in a table:
| Parameter | Testing Method | Typical Values | Challenges |
|---|---|---|---|
| Ionic Conductivity | EIS with blocking electrodes | 10⁻⁴ to 10⁻² S/cm | Sensitivity to pressure and surface conditions |
| Electronic Conductivity | DC polarization | <10⁻⁸ S/cm | Long stabilization times |
| Electrochemical Window | Linear sweep voltammetry (LSV) | Up to 5 V vs. Li/Li⁺ | Interface layer formation affecting stability |
| Mechanical Strength | Nanoindentation or tensile tests | Varies by material (e.g., sulfides: 1–10 GPa) | Brittleness and processing issues |
| Thermal Expansion | Dilatometry | Coefficient: 10⁻⁶ to 10⁻⁵ /°C | Mismatch with electrodes causing stress |
The electrochemical window is assessed using LSV, where the decomposition voltage $V_d$ is identified by a sudden current increase. However, in practical solid-state batteries, stable interface layers may form, allowing operation beyond the theoretical window. The mechanical properties, such as Young’s modulus $E$, are crucial for dendrite suppression and can be expressed as: $$E = \frac{\text{stress}}{\text{strain}}$$ High values of $E$ help prevent lithium dendrite penetration, enhancing cycle life.
Interface-Level Testing
Interfaces in solid-state batteries are prone to contact loss and side reactions. Techniques like in-situ computed tomography (CT) and electrochemical impedance spectroscopy (EIS) are used to monitor interface evolution. The impedance of an interface can be modeled using equivalent circuits, where the charge transfer resistance $R_{ct}$ reflects interface degradation. For example, the total impedance $Z$ might be represented as: $$Z = R_\Omega + \frac{1}{j\omega C_{dl} + \frac{1}{R_{ct}}}$$ where $R_\Omega$ is the ohmic resistance, $C_{dl}$ is the double-layer capacitance, and $\omega$ is the angular frequency. Table 2 summarizes common interface issues and testing methods:
| Interface Issue | Testing Technique | Key Metrics |
|---|---|---|
| Contact Loss | In-situ CT, ultrasound scanning | Area reduction, crack propagation |
| Side Reactions | XPS, Raman spectroscopy, EIS | Elemental diffusion, resistance increase |
| Lithium Dendrite Growth | SEM, AFM | Dendrite density, penetration depth |
In solid-state batteries, interface stability is critical for long-term performance, and advanced in-situ methods are needed to capture dynamic changes.
Single-Cell Level Testing
Testing at the single-cell level involves specialized fixtures to apply and maintain pressure, especially for sulfide-based solid-state batteries that require tens of MPa. Performance and lifespan tests must account for temperature, pressure, and current density interactions. The capacity retention over cycles can be modeled using empirical equations, such as: $$C(n) = C_0 \cdot e^{-\alpha n}$$ where $C(n)$ is the capacity at cycle $n$, $C_0$ is the initial capacity, and $\alpha$ is the degradation rate. Safety tests, including thermal runaway evaluations, are vital; the heat release rate $\dot{Q}$ during failure can be estimated as: $$\dot{Q} = I^2 \cdot R_{\text{internal}}$$ where $I$ is the current and $R_{\text{internal}}$ is the internal resistance. Table 3 outlines key single-cell tests:
| Test Category | Conditions | Metrics |
|---|---|---|
| Performance | Varied temperature (25–80°C), pressure (10–50 MPa) | Capacity, rate capability, efficiency |
| Lifespan | Cycling under accelerated stress (high C-rates) | Cycle life, capacity fade |
| Safety | Overcharge, short-circuit, thermal abuse | Onset temperature, gas emission |
For solid-state batteries, thermal stability is generally higher, but failure modes like internal short circuits due to dendrites require careful analysis.
System and Vehicle Level Testing
At the system level, solid-state batteries require integrated pressure management, thermal control, and battery management systems (BMS). The pressure $P$ must be uniformly maintained, which can be described by: $$P = \frac{F}{A}$$ where $F$ is the applied force and $A$ is the contact area. Thermal management systems must ensure rapid heating to optimal temperatures (e.g., 50–80°C for some solid-state batteries) and prevent hotspots. The energy density $\rho_E$ of a system can be calculated as: $$\rho_E = \frac{E_{\text{total}}}{V_{\text{system}}}$$ where $E_{\text{total}}$ is the total energy and $V_{\text{system}}$ is the system volume. Challenges include minimizing weight and volume while ensuring reliability under real-driving conditions, such as vibration and shock.
Challenges and Future Directions
The testing of solid-state batteries faces several challenges, including the need for in-situ characterization to observe real-time interface changes, intelligent simulation for predicting performance under complex conditions, and data-driven approaches for lifecycle analysis. For example, in-situ EIS can provide dynamic impedance data, while multiphysics models coupling electrochemistry, mechanics, and thermodynamics can simulate battery behavior. The use of machine learning algorithms can optimize test parameters and predict failures, enhancing the efficiency of evaluation systems.
In conclusion, constructing a robust testing and evaluation system for solid-state batteries is essential for their commercialization. By addressing the unique properties of solid-state batteries at all levels and advancing testing technologies, we can accelerate the development of safer and higher-performance energy storage solutions. The integration of in-situ, intelligent, and data-driven methods will play a key role in overcoming current limitations and unlocking the full potential of solid-state battery technology.
