As the world increasingly embraces environmental sustainability and green initiatives, the electric vehicle industry has experienced rapid growth. The performance of power batteries, as the core component of electric vehicles, directly determines key metrics such as driving range, charging speed, and safety. From early lead-acid batteries to the currently widely used lithium iron phosphate (LFP) and ternary lithium batteries, battery technology has continuously evolved. However, with rising market demands for higher performance in electric vehicles, the limitations of traditional liquid lithium-ion batteries in terms of energy density and safety have become more apparent. In this context, solid-state batteries have emerged as a promising next-generation battery technology due to their unique advantages, attracting widespread attention and research. In this article, I will explore the principles, structure, development status, advantages, and challenges of solid-state batteries, emphasizing their potential to revolutionize the electric vehicle sector. Throughout this discussion, I will use tables and equations to summarize key points, ensuring a comprehensive analysis that highlights the importance of solid-state battery innovations.
Solid-state batteries operate on a similar charge-discharge mechanism as traditional liquid lithium-ion batteries, both relying on the intercalation and deintercalation of lithium ions between the positive and negative electrodes. During charging, lithium ions detach from the positive electrode material, migrate through the electrolyte, and embed into the negative electrode; the reverse occurs during discharge. The key difference lies in the physical state of the electrolyte: solid-state batteries use a solid electrolyte instead of a liquid one to facilitate ion conduction. This solid electrolyte not only enables efficient lithium-ion migration but also exhibits excellent electronic insulation, reducing the risk of internal short circuits. Moreover, the chemical stability of solid electrolytes surpasses that of liquid electrolytes, leading to more stable lithium-ion transport paths and minimizing performance degradation and safety hazards caused by issues like electrolyte evaporation or leakage. The fundamental equation governing ion transport can be expressed using the Nernst-Planck equation for ion flux, which in its simplified form for solid-state batteries is: $$ J = -D \frac{\partial C}{\partial x} + \frac{zF}{RT} D C \frac{\partial \phi}{\partial x} $$ where \( J \) is the ion flux, \( D \) is the diffusion coefficient, \( C \) is the ion concentration, \( x \) is the spatial coordinate, \( z \) is the charge number, \( F \) is Faraday’s constant, \( R \) is the gas constant, \( T \) is the temperature, and \( \phi \) is the electric potential. This equation highlights how solid electrolytes can maintain stable ion migration under varying conditions, a critical factor for solid-state battery performance.
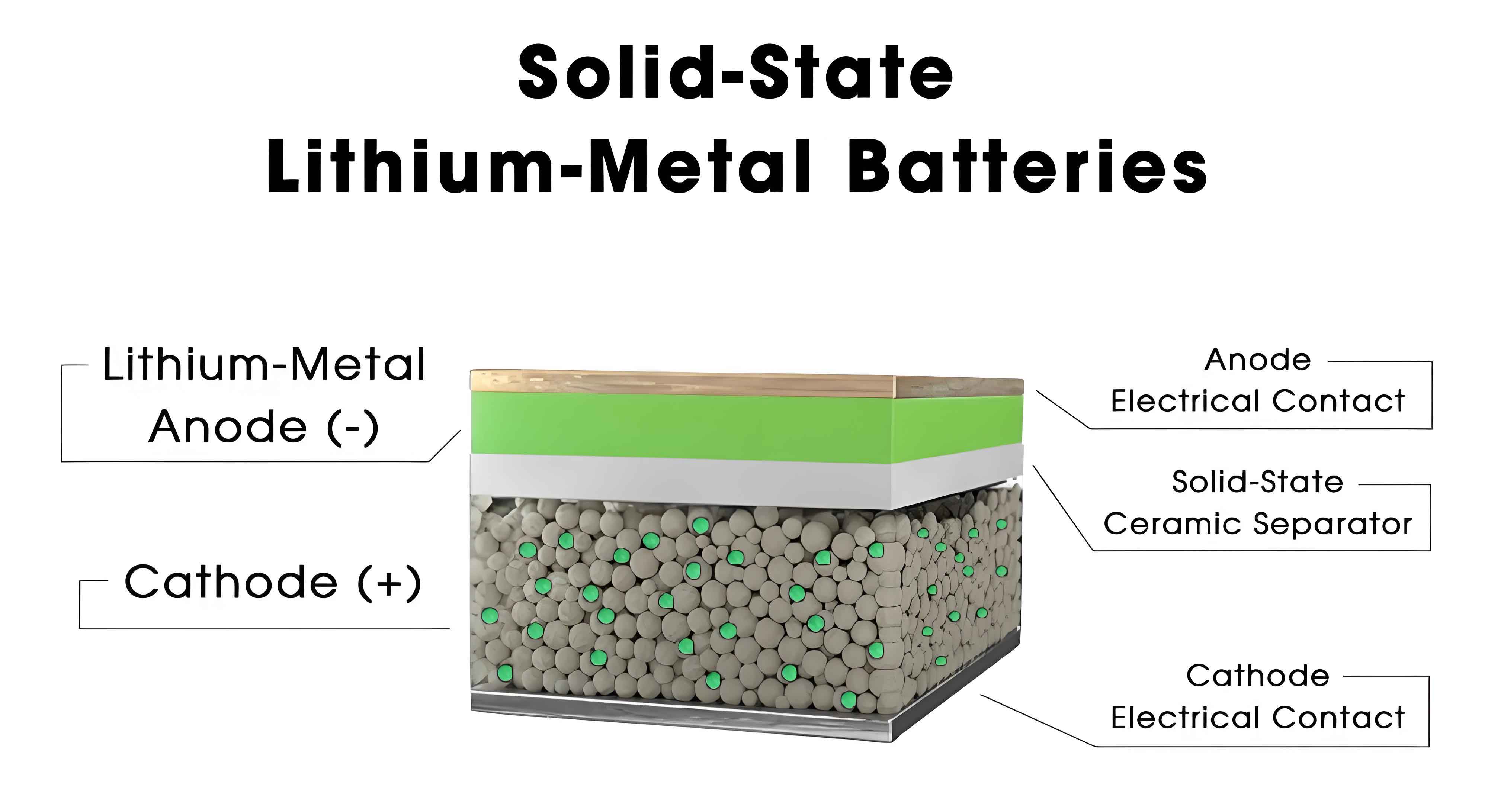
The structure of a solid-state battery consists of key materials including the positive electrode, negative electrode, and solid electrolyte, which replace the liquid components found in traditional batteries. The positive electrode materials typically include layered oxides (e.g., LiCoO₂), polyanion compounds (e.g., LiFePO₄), and lithium-rich manganese-based materials. These materials determine the lithium-ion storage capacity and electrochemical performance, with their crystal structures influencing the battery’s overall efficiency. For instance, the capacity of a positive electrode can be modeled by: $$ Q = nF \cdot \Delta x $$ where \( Q \) is the capacity, \( n \) is the number of electrons transferred, \( F \) is Faraday’s constant, and \( \Delta x \) is the change in lithium concentration. The negative electrode materials encompass carbon-based materials (e.g., graphite), metallic lithium, lithium alloys, and silicon-based materials. Metallic lithium anodes offer a high theoretical specific capacity of 3,860 mAh/g, but they are prone to lithium dendrite growth, which can penetrate the electrolyte and cause short circuits. To mitigate this, the use of solid electrolytes in solid-state batteries helps suppress dendrite formation, enhancing safety. The solid electrolyte is the core component, categorized into inorganic types (e.g., oxide-based like Li₇La₃Zr₂O₁₂, sulfide-based like Li₃PS₄, and halide-based) and organic types (e.g., poly(ethylene oxide) or PEO-based materials). These electrolytes must exhibit high ionic conductivity, chemical stability, compatibility with electrode materials, and good mechanical properties. The ionic conductivity \( \sigma \) can be described by: $$ \sigma = n \cdot e \cdot \mu $$ where \( n \) is the charge carrier concentration, \( e \) is the elementary charge, and \( \mu \) is the mobility. Additionally, current collectors, typically made of aluminum foil for the positive electrode and copper foil for the negative electrode, play a crucial role in collecting and conducting current, with their properties affecting internal resistance and charge-discharge performance. The table below summarizes the common materials used in solid-state batteries and their key characteristics, emphasizing the diversity and advantages of solid-state battery components.
| Component | Material Types | Key Properties | Challenges |
|---|---|---|---|
| Positive Electrode | Layered Oxides (e.g., LiCoO₂), Polyanion Compounds (e.g., LiFePO₄), Lithium-Rich Manganese-Based | High capacity, structural stability | Interface compatibility with solid electrolyte |
| Negative Electrode | Graphite, Metallic Lithium, Silicon-Based | High theoretical capacity (e.g., 3,860 mAh/g for Li) | Lithium dendrite growth, volume expansion |
| Solid Electrolyte | Oxide (e.g., Li₇La₃Zr₂O₁₂), Sulfide (e.g., Li₃PS₄), Halide, Polymer (e.g., PEO) | Ionic conductivity, electronic insulation, thermal stability | Low room-temperature conductivity for some types |
| Current Collectors | Aluminum (positive), Copper (negative) | High conductivity, thin films | Interface resistance with electrodes |
In terms of development, solid-state batteries have seen significant research and industrialization progress globally. Research efforts have focused on overcoming key bottlenecks, such as developing novel positive and negative electrode materials and solid electrolytes to enhance energy density, cycle life, and safety. For example, in solid electrolytes, structural design and surface modifications of sulfide-based materials have improved ionic conductivity and air stability. Similarly, strategies for protecting lithium metal anodes have been explored to inhibit dendrite growth. On the manufacturing front, companies have developed scalable production techniques like coating and hot-pressing to fabricate electrode and electrolyte layers, boosting efficiency and consistency. The industrialization process has achieved milestones, with international players like Toyota planning to launch electric vehicles equipped with proprietary solid-state batteries that offer superior energy density and charging rates. In China, firms such as CATL and Ganfeng Lithium are advancing their solid-state battery initiatives through dedicated production lines and pilot projects. However, large-scale commercialization faces hurdles, including high costs due to scarce raw materials and complex processes, as well as challenges in maintaining production consistency. The table below provides an overview of the global solid-state battery development status, highlighting the ongoing efforts and barriers in bringing solid-state batteries to market.
| Region/Company | Key Developments | Target Applications | Challenges |
|---|---|---|---|
| Japan (e.g., Toyota) | Plans for EV launch with solid-state batteries, high energy density prototypes | Electric vehicles, consumer electronics | Cost reduction, scalability |
| China (e.g., CATL, Ganfeng) | Pilot production lines, integration with EV platforms | Automotive, energy storage | Interface stability, mass production |
| Europe/US (e.g., startups, universities) | Research on novel electrolytes, partnerships with automakers | Next-generation EVs, aerospace | Ionic conductivity, funding |
| Global Research Institutions | Advances in sulfide and polymer electrolytes, dendrite suppression | Broad applications | Technology transfer to industry |
The advantages of solid-state batteries are substantial, positioning them as a transformative technology for electric vehicles. First, they offer higher energy density compared to traditional batteries. By replacing liquid electrolytes with solid ones, solid-state batteries reduce the proportion of inactive materials, allowing more space for active electrodes and increasing overall energy density. Theoretically, solid-state batteries can achieve energy densities of 400–500 Wh/kg, whereas lithium iron phosphate batteries typically range from 140–200 Wh/kg, and ternary lithium batteries from 200–300 Wh/kg. This higher energy density translates to longer driving ranges for electric vehicles, alleviating range anxiety. The energy density \( E_d \) can be calculated as: $$ E_d = \frac{Q \cdot V}{m} $$ where \( Q \) is the capacity, \( V \) is the voltage, and \( m \) is the mass. For solid-state batteries, improvements in electrode materials and electrolyte design contribute to higher \( Q \) and \( V \), resulting in superior \( E_d \). Second, safety is enhanced in solid-state batteries. Traditional liquid lithium-ion batteries are prone to thermal runaway due to flammable electrolytes, but solid electrolytes are non-flammable and thermally stable, reducing risks of fire or explosion. Moreover, solid-state batteries effectively suppress lithium dendrite growth, preventing short circuits. Third, solid-state batteries exhibit longer cycle life. The stable interfaces between solid electrolytes and electrodes minimize structural changes and active material loss during cycling, enabling lifetimes exceeding 3,000 cycles. In contrast, LFP batteries last about 2,000–3,000 cycles, and ternary lithium batteries 1,500–2,500 cycles. This extended lifespan reduces replacement costs and improves economic viability. The cycle life \( N \) can be modeled using empirical equations like: $$ N = N_0 \cdot \exp\left(-\frac{E_a}{RT}\right) $$ where \( N_0 \) is a pre-exponential factor, \( E_a \) is the activation energy for degradation, \( R \) is the gas constant, and \( T \) is the temperature. Solid-state batteries typically have higher \( E_a \) values, indicating slower degradation. Fourth, solid-state batteries operate over a wider temperature range. Unlike liquid electrolytes, which suffer from increased viscosity at low temperatures and thermal instability at high temperatures, solid electrolytes maintain stable ionic conduction across diverse conditions. This improves the reliability of electric vehicles in extreme climates. The table below compares the performance metrics of solid-state batteries with traditional lithium-ion batteries, underscoring the benefits of solid-state technology.
| Parameter | Solid-State Batteries | Lithium Iron Phosphate (LFP) | Ternary Lithium Batteries |
|---|---|---|---|
| Energy Density (Wh/kg) | 400–500 (theoretical) | 140–200 | 200–300 |
| Cycle Life (cycles) | >3,000 | 2,000–3,000 | 1,500–2,500 |
| Safety | High (non-flammable electrolyte) | Moderate | Lower (flammable electrolyte) |
| Operating Temperature Range | -40°C to 100°C+ (estimated) | -20°C to 60°C | -20°C to 60°C |
| Ionic Conductivity (S/cm) at 25°C | 10⁻⁴ to 10⁻² (varies by material) | ~10⁻² (liquid electrolyte) | ~10⁻² (liquid electrolyte) |
Despite these advantages, solid-state batteries face several challenges that hinder their widespread adoption. One major issue is high cost. The production of solid-state batteries involves expensive raw materials, such as high-purity solid electrolytes, and complex manufacturing processes like precise layer deposition and hot-pressing. For instance, sulfide-based solid electrolytes can be costly due to rare elements and synthesis difficulties. The cost per kWh for solid-state batteries is currently higher than for LFP or ternary lithium batteries, which benefit from mature supply chains. A simplified cost model can be expressed as: $$ C_{\text{total}} = C_{\text{materials}} + C_{\text{manufacturing}} + C_{\text{R&D}} $$ where \( C_{\text{total}} \) is the total cost, and solid-state batteries have elevated \( C_{\text{materials}} \) and \( C_{\text{manufacturing}} \) due to technical hurdles. Second, ionic conductivity remains a concern. While some solid electrolytes show high conductivity, others, particularly at room temperature, have lower values than liquid electrolytes (e.g., <10⁻³ S/cm for certain oxides), leading to increased internal resistance and reduced charge-discharge rates. The conductivity \( \sigma \) can be improved by optimizing material composition, as shown in the equation: $$ \sigma = \sigma_0 \exp\left(-\frac{E_a}{kT}\right) $$ where \( \sigma_0 \) is a pre-exponential factor, \( E_a \) is the activation energy for conduction, \( k \) is Boltzmann’s constant, and \( T \) is the temperature. Research aims to lower \( E_a \) for solid electrolytes to enhance performance. Third, interface compatibility poses a significant challenge. The solid-solid interfaces between electrolytes and electrodes can lead to high interfacial resistance, stress concentrations, and chemical reactions during cycling, impairing battery efficiency and longevity. Strategies like surface coatings and composite materials are being developed to address this. Fourth, manufacturing processes for solid-state batteries are complex and not yet fully optimized for mass production. Techniques such as thin-film deposition and lamination require precise control over thickness and uniformity, increasing equipment costs and reducing yield rates. The table below summarizes these challenges and potential solutions, illustrating the ongoing efforts to overcome the limitations of solid-state batteries.
| Challenge | Description | Potential Solutions | Impact on Commercialization |
|---|---|---|---|
| High Cost | Expensive materials and processes | Develop low-cost electrolytes, scale production | Slows market penetration |
| Low Ionic Conductivity | Reduced ion transport at room temperature | Material doping, nanostructuring | Affects power density and charging speed |
| Interface Compatibility | High resistance and degradation at interfaces | Interface engineering, protective layers | Limits cycle life and performance |
| Complex Manufacturing | Multi-step processes with high precision | Automation, advanced coating techniques | Increases production time and cost |
In conclusion, solid-state batteries represent a groundbreaking advancement in the electric vehicle battery sector, with their high energy density, enhanced safety, long cycle life, and broad operating temperature range offering innovative solutions to current limitations like range anxiety and safety concerns. However, challenges such as high costs, ionic conductivity issues, interface compatibility, and complex manufacturing processes must be addressed through continued research and development. As technology progresses and industrialization scales up, solid-state batteries have the potential to achieve large-scale commercialization, driving the electric vehicle industry toward greater efficiency and sustainability. Looking ahead, the evolution of solid-state battery technology will likely complement existing battery systems, creating a more robust energy storage ecosystem that meets the growing demand for clean and reliable transportation. The journey toward widespread adoption of solid-state batteries is fraught with obstacles, but the promise they hold for a greener future makes them a critical focus for innovation in the years to come.
Throughout this discussion, I have emphasized the transformative potential of solid-state batteries, and it is clear that overcoming the existing hurdles will require collaborative efforts across academia and industry. By leveraging advanced materials science and engineering, we can unlock the full capabilities of solid-state batteries, ultimately contributing to global efforts in reducing carbon emissions and promoting sustainable mobility. The repeated mention of solid-state battery and solid-state batteries in this article underscores their centrality to the future of energy storage, and I am optimistic that with persistent innovation, these batteries will become a cornerstone of next-generation electric vehicles.
