The science behind lithium-ion (Li-ion) batteries encompasses a fascinating blend of chemistry, physics, and engineering. These batteries have become ubiquitous in our daily lives, powering everything from mobile phones to electric vehicles (EVs) and large-scale energy storage systems. Understanding the principles that govern their operation is crucial for appreciating their efficiency, capabilities, and the challenges they face. This article explores the fundamental science behind lithium-ion batteries, including their components, operation, and the innovations that continue to enhance their performance.
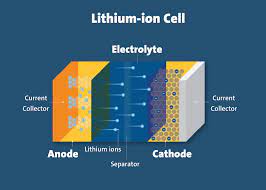
Basic Components of a Lithium-Ion Battery
A lithium-ion battery consists of three primary components: the anode (negative electrode), the cathode (positive electrode), and the electrolyte. The anode is typically made from carbon-based materials like graphite, while the cathode is composed of lithium metal oxides, such as lithium cobalt oxide (LiCoO2) or lithium iron phosphate (LiFePO4). The electrolyte is a lithium salt dissolved in an organic solvent, and it facilitates the movement of lithium ions between the anode and cathode.
How Lithium-Ion Batteries Work
The operation of a lithium-ion battery is based on the movement of lithium ions between the anode and cathode during charging and discharging cycles. When the battery is charging, lithium ions move from the cathode through the electrolyte and insert themselves into the anode. This process stores energy in the battery. During discharge, the lithium ions move back to the cathode, releasing the stored energy as electrical power.
The Role of the Electrolyte and Separator
The electrolyte plays a crucial role in the operation of lithium-ion batteries. It not only facilitates the movement of lithium ions between the electrodes but also participates in the formation of a solid electrolyte interphase (SEI) on the anode surface. This SEI layer is crucial for the battery’s longevity and performance, protecting the anode and allowing lithium ions to pass through while preventing electron flow.
A separator is placed between the anode and cathode to prevent direct contact and potential short-circuiting. This separator is porous, allowing lithium ions to pass through while maintaining electrical insulation between the electrodes.
Energy Density and Efficiency
The energy density of a lithium-ion battery, which is the amount of energy it can store per unit weight or volume, is significantly higher than that of other types of rechargeable batteries. This high energy density is a result of the lightweight nature of lithium and the battery’s ability to hold a large number of lithium ions. Additionally, Li-ion batteries have a high efficiency, meaning that a large percentage of the energy stored during charging can be retrieved during discharge.
Challenges and Innovations
While lithium-ion batteries offer numerous advantages, they also face challenges such as aging, capacity loss over time, safety risks associated with overheating and thermal runaway, and the environmental impact of lithium mining and battery disposal. Innovations in battery technology aim to address these challenges by developing new materials for anodes, cathodes, and electrolytes; enhancing battery management systems for improved safety; and researching recycling methods to recover valuable materials from used batteries.
Future Directions
Research into improving lithium-ion batteries continues to push the boundaries of energy storage technology. Innovations in solid-state batteries, which replace the liquid electrolyte with a solid electrolyte, promise to improve safety and potentially increase energy density. Other areas of research include developing alternative anode materials, such as silicon, which could significantly increase the battery’s capacity, and exploring new cathode materials that are less expensive and more environmentally friendly.
Conclusion
The science behind lithium-ion batteries is a testament to the remarkable advancements in materials science and electrochemistry. As research continues to evolve, we can expect to see further improvements in battery performance, safety, and sustainability, ensuring that lithium-ion batteries remain at the forefront of energy storage technology.
