In recent years, I have observed a significant surge in the production and sales of new energy vehicles, including pure electric and hybrid models, leading to a remarkable increase in market penetration. However, the widespread adoption of these vehicles continues to face numerous challenges, with battery technology being a primary bottleneck. Traditional liquid batteries, such as lithium-ion variants, exhibit inherent limitations in energy density, safety performance, service life, and charging efficiency, which often fall short of meeting the escalating performance standards and consumer expectations in the new energy vehicle market. As an alternative, solid-state batteries have garnered considerable attention due to their superior attributes in energy density, charge-discharge efficiency, thermal stability, and safety, making them highly suitable for the stringent requirements of new energy vehicles regarding range, charging time, and overall reliability. In this article, I will delve into the development of solid-state battery technology, explore its current applications and advantages in new energy vehicles, and discuss the latest research progress in solid-state electrolytes and electrode materials. Additionally, I will highlight specific use cases and propose future directions for the automotive industry and policymakers to foster innovation and infrastructure development, thereby accelerating the progress of the new energy vehicle sector.
The evolution of solid-state batteries represents a pivotal shift in energy storage solutions. Solid-state batteries utilize solid electrolytes instead of liquid ones, facilitating ion migration through solid lattice structures and enabling efficient energy storage and release. During charging, cations within the solid electrolyte migrate from the positive electrode to the negative electrode under an electric field, overcoming energy barriers in the lattice and integrating with the negative electrode material. Conversely, during discharge, these cations reverse their path, moving back to the positive electrode while electrons flow through an external circuit, completing the energy release process. This mechanism not only enhances safety by eliminating flammable liquid components but also improves energy density and cycle life. The core components of solid-state batteries include various types of solid electrolytes and innovative electrode materials, which I will elaborate on in subsequent sections. The growing interest in solid-state batteries stems from their potential to address critical issues like thermal runaway and limited range, which are prevalent in conventional lithium-ion batteries. For instance, in high-temperature conditions or internal short circuits, liquid batteries may experience thermal instability, leading to hazardous events, whereas solid-state batteries offer inherent stability due to their solid structure.
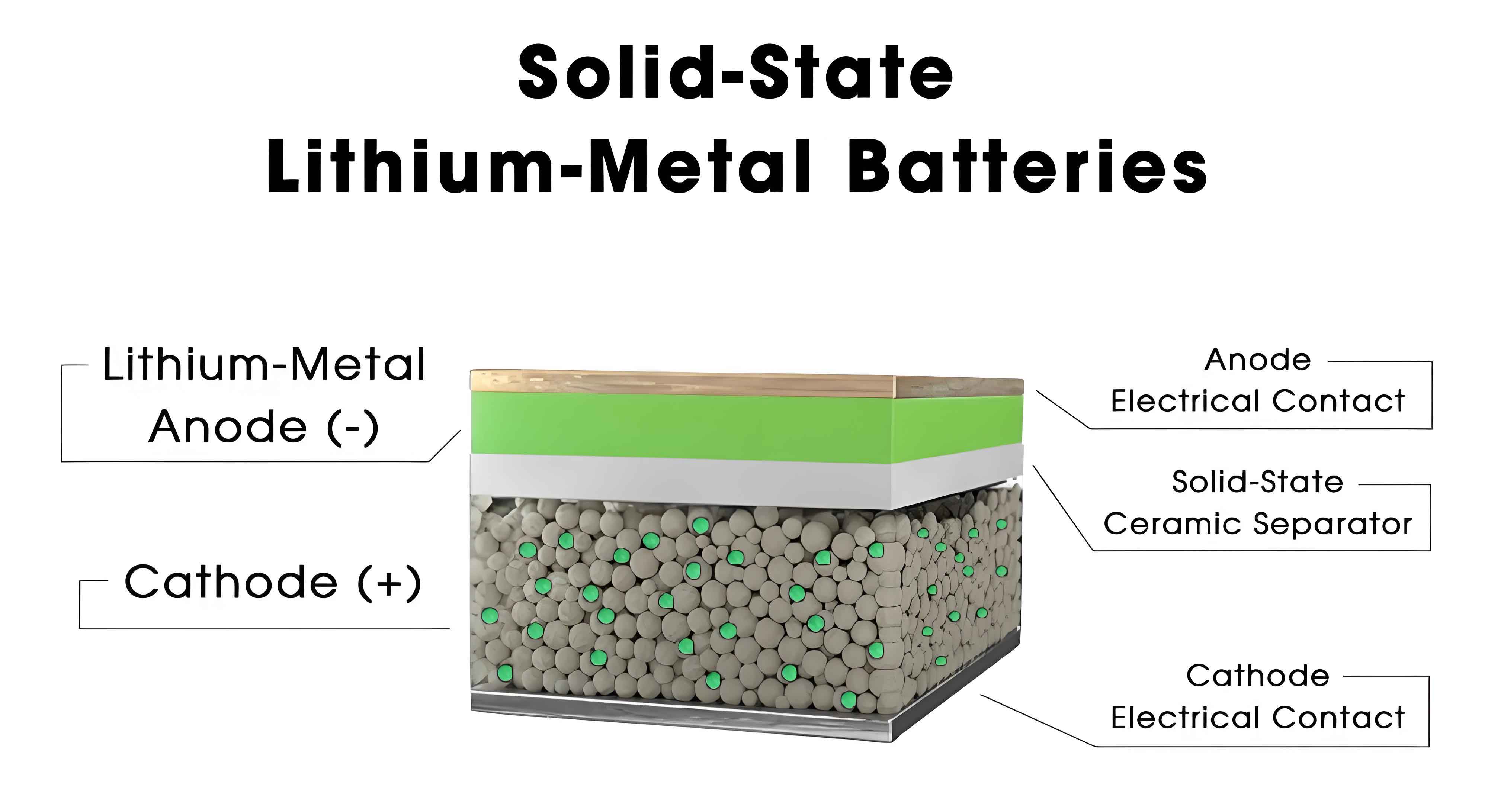
Solid-state batteries can be classified based on the materials used for their solid electrolytes, each with distinct properties and applications. The primary categories include oxide electrolytes, sulfide electrolytes, polymer electrolytes, and halide electrolytes. Oxide electrolytes, such as NASICON-type, perovskite-type, garnet-type, and lithium superionic conductors, are known for their high ionic conductivity, mechanical strength, air stability, and wide electrochemical windows. For example, lithium lanthanum zirconate (LLZO) exhibits an ionic conductivity of up to $$10^{-3} \, \text{S/cm}$$ at room temperature, which can be represented by the equation for ionic conduction: $$\sigma = n \cdot e \cdot \mu$$, where $\sigma$ is conductivity, $n$ is the charge carrier density, $e$ is the elementary charge, and $\mu$ is the mobility. However, challenges like grain boundary resistance often reduce overall performance, prompting research into synthesis methods like sol-gel and solid-state reactions to optimize these materials. Sulfide electrolytes, including LPS-type (thiophosphates), Argyrodite-type, LGPS-type, and Thio-LISICON-type, offer even higher ionic conductivities, reaching up to $$10^{-2} \, \text{S/cm}$$, due to their soft and deformable nature that ensures good interfacial contact. Despite this, sulfide-based solid-state batteries suffer from poor air stability and chemical reactivity, necessitating doping with elements like nitrogen or boron to enhance durability. Polymer electrolytes, such as polystyrene sulfonate lithium (PSS-Li), provide flexibility and ease of processing but typically have lower ionic conductivities in the range of $$10^{-6} \, \text{to} \, 10^{-4} \, \text{S/cm}$$. To overcome this, composite approaches incorporating inorganic fillers have been developed to boost conductivity. Halide electrolytes, including those based on group 3 metals (e.g., Sc, Y) and group 4 metals (e.g., Zr, Hf), demonstrate high ionic conductivities (e.g., $$10^{-3} \, \text{S/cm}$$ for Li₂ZrCl₆) and excellent stability, though their high cost remains a barrier to widespread adoption. The diversity in solid electrolyte materials underscores the ongoing research efforts to balance performance, safety, and economic feasibility for solid-state batteries in commercial applications.
The historical development of solid-state battery technology dates back to the 1970s, with early research focusing on exploring solid electrolyte materials. In 1980, a team from a Japanese university proposed the use of lithium fluoride and hydrogen fluoride compounds as potential solid electrolytes, laying the groundwork for future advancements. By 1991, collaborative efforts between industry and academia led to the introduction of multilayer solid-state battery prototypes with improved charging efficiency. The 21st century witnessed accelerated progress, with key material optimizations significantly enhancing battery performance. For instance, in 2010, composite materials combining oxides and polymers gained attention for their ability to improve low-temperature performance, while in 2015, a major automotive company announced breakthroughs achieving energy densities of 7.5 mAh/cm² at room temperature. More recently, in 2024, innovations by companies like TaiLan New Energy resulted in all-solid-state lithium metal batteries with energy densities as high as 720 Wh/kg, utilizing high-capacity lithium-rich manganese-based cathodes and composite lithium metal anodes. This achievement not only set industry records but also addressed fundamental concerns regarding range and safety in electric vehicles. Similarly, other industry leaders have invested heavily in research, overcoming interface challenges to achieve cycle lives exceeding 2000 cycles. The current landscape indicates a compound annual growth rate of over 30% for the solid-state battery market between 2025 and 2030, driven by increasing investments and technological refinements. However, challenges such as the ionic conductivity of solid electrolytes, interface stability, and manufacturing scalability persist, requiring continued innovation in materials science and electrochemistry.
In terms of key materials, solid electrolytes play a central role in determining the overall performance of solid-state batteries. Oxide electrolytes, like LLZO and LTO, are prized for their chemical and thermal stability, with ionic conductivities that can be optimized through advanced fabrication techniques. The conductivity can be modeled using the Arrhenius equation: $$\sigma = \sigma_0 \exp\left(-\frac{E_a}{kT}\right)$$, where $\sigma_0$ is the pre-exponential factor, $E_a$ is the activation energy, $k$ is Boltzmann’s constant, and $T$ is temperature. Sulfide electrolytes, such as the Li₂S-P₂S₅ system, exhibit superior ionic conductivities but are sensitive to moisture, leading to degradation. Research into composite materials and elemental doping has shown promise in mitigating these issues. Polymer electrolytes, while flexible and safe, often require enhancements through nanocomposites to achieve practical conductivity levels. For example, incorporating ceramic nanoparticles into polymer matrices can increase ionic transport by creating percolation pathways, as described by the equation for composite conductivity: $$\sigma_{\text{composite}} = \phi \sigma_{\text{filler}} + (1-\phi) \sigma_{\text{matrix}}$$, where $\phi$ is the volume fraction of the filler. Halide electrolytes, though less common, offer a balance of high conductivity and stability, but cost considerations limit their current applicability.
Innovations in electrode materials are equally critical for advancing solid-state batteries. For anode materials, silicon and its alloys stand out due to their high theoretical capacity of 4200 mAh/g. However, the substantial volume expansion during cycling poses a challenge, which can be addressed through nanostructuring, carbon coating, and composite formation. For instance, encapsulating silicon nanoparticles in conductive carbon layers not only improves electronic conductivity but also accommodates volume changes, extending cycle life. The capacity retention can be expressed as: $$C_r = \frac{C_{\text{after}}}{C_{\text{initial}}} \times 100\%$$, where $C_r$ is the retention percentage, and $C_{\text{after}}$ and $C_{\text{initial}}$ are capacities after and before cycling, respectively. For cathode materials, high-capacity options like lithium-rich layered oxides (e.g., NMC) and sulfur-based compounds are being developed. Doping with elements such as nickel or manganese enhances lithium-ion intercalation, increasing specific capacity. Nanostructuring, such as using LiCoO₂ nanowires, shortens ion diffusion paths, improving reaction kinetics. Surface modifications, including coatings with ion-conductive layers, further enhance interface stability and high-rate performance. High-entropy alloys and advanced composites are also being explored to boost high-temperature stability, enabling operation up to 350°C. The continuous refinement of these materials is essential for realizing the full potential of solid-state batteries in demanding applications like electric vehicles.
| Electrolyte Type | Ionic Conductivity (S/cm) | Advantages | Disadvantages | Common Materials |
|---|---|---|---|---|
| Oxide | $$10^{-3} \, \text{to} \, 10^{-2}$$ | High stability, wide electrochemical window | Grain boundary resistance | LLZO, LTO |
| Sulfide | $$10^{-2} \, \text{to} \, 10^{-1}$$ | High conductivity, good interface contact | Moisture sensitivity, chemical instability | Li₂S-P₂S₅, LGPS |
| Polymer | $$10^{-6} \, \text{to} \, 10^{-4}$$ | Flexibility, ease of processing | Low conductivity, mechanical weakness | PSS-Li, PEO-based |
| Halide | $$10^{-3} \, \text{to} \, 10^{-2}$$ | High conductivity, good stability | High cost, limited practicality | Li₂ZrCl₆, Sc-based halides |
The application of solid-state batteries in new energy vehicles has gained momentum, driven by their exceptional energy density, safety, and charging capabilities. For example, solid-state batteries can achieve energy densities exceeding 500 Wh/kg, significantly enhancing vehicle range. The energy density can be calculated as: $$E_d = \frac{Q \cdot V}{m}$$, where $E_d$ is energy density, $Q$ is charge capacity, $V$ is voltage, and $m$ is mass. In terms of safety, the absence of liquid electrolytes reduces risks of leakage and thermal runaway, with studies showing that solid-state batteries can withstand temperatures up to 200°C higher than traditional lithium-ion batteries before failure. Charging efficiency is another forte, with some solid-state batteries supporting rates up to 5C, enabling rapid charging times—for instance, adding over 400 km of range in just 12 minutes. Major automotive manufacturers have integrated solid-state battery technology into their models. For instance, the IM L6 sedan features a “light-year solid-state battery” with an energy density over 300 Wh/kg, enabling a range of more than 1000 km. Similarly, GAC Aion’s in-situ grown solid electrolyte interface technology has pushed energy densities beyond 350 Wh/kg, while CATL’s condensed-state electrolyte batteries achieve up to 500 Wh/kg with improved thermal stability. Collaborations, such as that between Chery Auto and TaiLan New Energy, focus on developing separator-free solid-state lithium batteries targeting 600 Wh/kg through nano-scale ion channel control and 3D conductive networks. Internationally, companies like Nissan and Toyota are advancing sulfide-based solid electrolytes, with plans to launch mass-produced electric vehicles equipped with all-solid-state batteries by 2027-2028. These developments not only enhance vehicle performance but also contribute to reducing the carbon footprint of transportation, aligning with global sustainability goals.
Despite the promising advancements, the widespread adoption of solid-state batteries faces several challenges. Economically, the high cost of raw materials and complex manufacturing processes, such as thin-film deposition and interface engineering, hinder scalability. The cost per kWh for solid-state batteries currently exceeds that of liquid lithium-ion batteries, but economies of scale and technological improvements are expected to narrow this gap. Technically, issues like interfacial resistance between solid components and limited cycle life under high loads require further research. For example, the interfacial resistance can be modeled as: $$R_{\text{interface}} = \frac{\delta}{\sigma_{\text{interface}}}$$, where $\delta$ is the interface thickness and $\sigma_{\text{interface}}$ is its conductivity. Additionally, infrastructure gaps, such as insufficient charging and swapping facilities, pose barriers to the seamless integration of electric vehicles powered by solid-state batteries. From a policy perspective, increased investment in R&D and public infrastructure is crucial. I believe that collaborative efforts between industry and government can accelerate innovation, for instance, through subsidies for research consortia and the development of smart grids to support fast-charging networks. Looking ahead, the potential for solid-state batteries to revolutionize the energy storage landscape is immense, with ongoing research focusing on hybrid systems and recycling methods to enhance sustainability.
In conclusion, solid-state batteries represent a transformative technology for new energy vehicles, offering superior energy density, safety, and efficiency compared to conventional options. Through my analysis, I have highlighted the progress in solid electrolyte and electrode materials, as well as the practical applications in vehicles that demonstrate extended range and faster charging. However, challenges related to cost, manufacturing, and infrastructure must be addressed to fully realize their potential. I urge the automotive industry to intensify R&D efforts in solid-state battery technology, particularly in materials science and production techniques, while policymakers should prioritize the expansion of charging infrastructure and supportive regulations. As global demand for clean transportation grows, the continued evolution of solid-state batteries will play a pivotal role in achieving a sustainable and energy-efficient future, ultimately driving the transition away from fossil fuels and toward a greener automotive ecosystem.
