Abstract
This study examines the thermal runaway behavior of high-capacity (120 Ah) lithium iron phosphate (LiFePO4) batteries under different test atmospheres, specifically inert (nitrogen) and air. The batteries were subjected to thermal abuse in a sealed pressure chamber, and their body and ambient temperatures, as well as gas composition, production, exhaust rate, and flammability limits, were analyzed. Comparative experiments under both atmospheres revealed that the presence of air significantly influenced the thermal runaway process, leading to an increase in temperature, duration, and gas production. The findings offer valuable insights into the safety concerns and mitigation strategies for large-scale LiFePO4 battery systems.
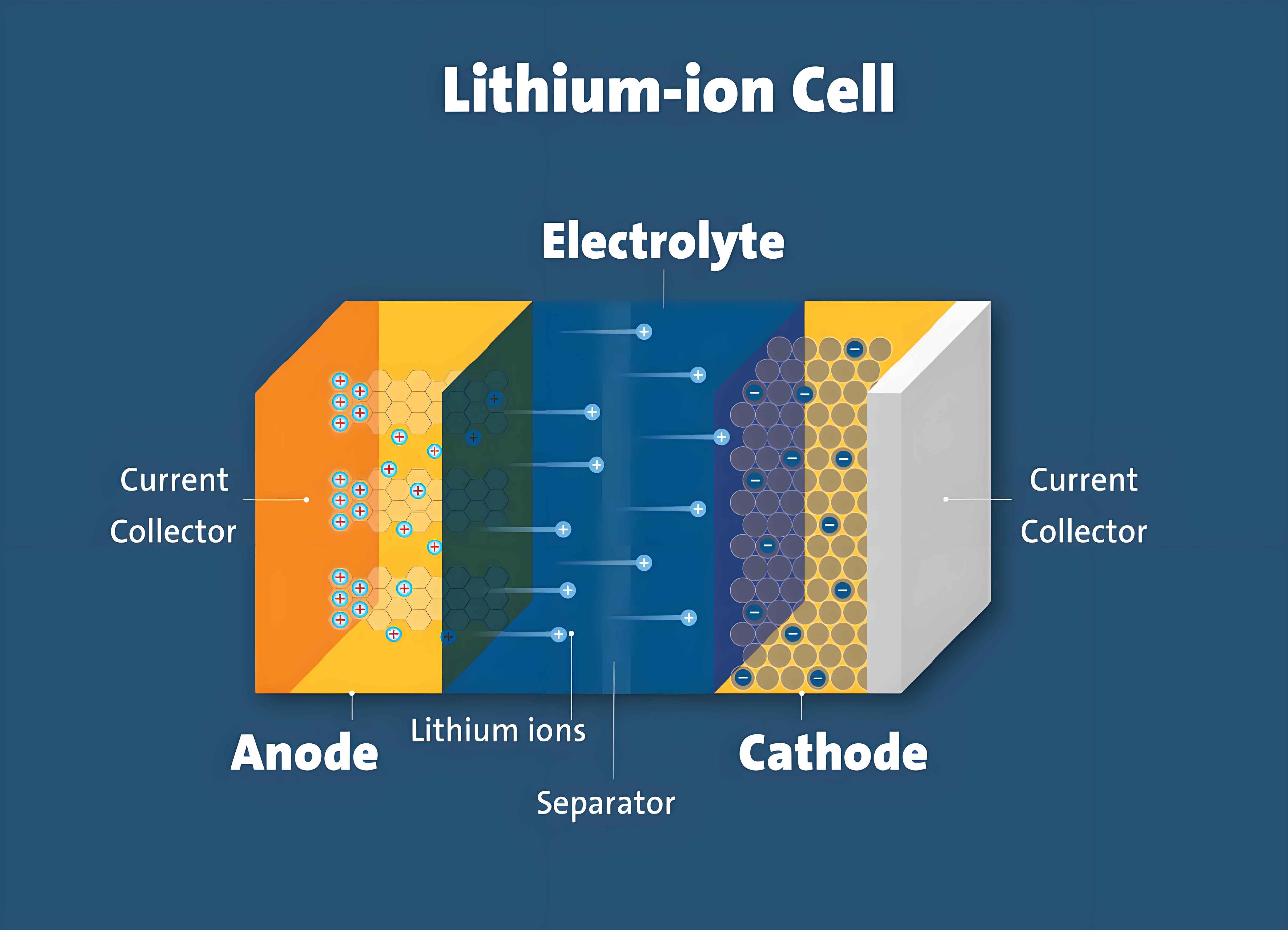
1. Introduction
Lithium-ion batteries (LIBs) have become ubiquitous in various applications due to their high energy density, long cycle life, and environmental friendliness. However, their safety remains a significant concern, especially under conditions of thermal or electrical abuse. Thermal runaway, a spontaneous and exothermic chain reaction within the battery, can result in catastrophic failures such as fires and explosions. This study focuses on the thermal runaway behavior of large-capacity LiFePO4 batteries under different test atmospheres, aiming to provide a deeper understanding of the underlying mechanisms and guide the development of safer battery systems.
2. Literature Review
Previous studies have extensively investigated the thermal runaway behavior of LIBs, focusing on factors such as state of charge (SoC), battery chemistry, and heating methods. However, most of these studies have concentrated on smaller capacity batteries or batteries with different cathode chemistries. Research specifically addressing the effect of test atmosphere on the thermal runaway behavior of high-capacity LiFePO4 batteries is limited.
2.1 Thermal Runaway Mechanisms
Thermal runaway in LIBs typically involves several stages, including heating, venting, thermal runaway, and subsequent cooling. This complex chain reaction is triggered by internal short circuits, overcharging, or external heating, leading to a rapid increase in temperature and the generation of flammable gases.
2.2 Influence of Test Atmosphere
The test atmosphere can significantly impact the thermal runaway behavior of LIBs. For instance, the presence of oxygen in the air can accelerate chemical reactions within the battery, leading to higher temperatures and increased gas production. However, the specific effects on large-capacity LiFePO4 batteries have not been thoroughly explored.
3. Experimental Methodology
3.1 Test Battery
The subject of this study was a 120 Ah square-shaped LiFePO4 battery with LiFePO4 as the cathode material and graphite as the anode. Key battery parameters are summarized in Table 1.
Table 1: Battery Basic Performance Parameters
| Parameter | Value |
|---|---|
| Cathode Material | LiFePO4 |
| Anode Material | Graphite |
| Rated Capacity (Ah) | 120 |
| Dimensions (mm) | 174 x 170 x 48 |
| Initial Mass (g) | 2860 |
| Nominal Voltage (V) | 3.20 |
| Cut-off Voltage (V) | 2.50 – 3.65 |
3.2 Experimental Setup
The experiments were conducted in a sealed pressure chamber with a volume of 82 liters. The battery was heated laterally using a 952W heating plate to induce thermal runaway. Sensors were placed at strategic locations on the battery surface and surroundings to monitor temperature and pressure changes.
3.3 Testing Procedures
The battery was first discharged to 2.50 V and then charged to 100% SoC before being subjected to thermal abuse. Experiments were performed under both inert (nitrogen) and air atmospheres, with each condition repeated twice for consistency. Gas samples were collected and analyzed using gas chromatography to determine composition and flammability limits.
4. Results and Discussion
4.1 Battery Surface Temperature
The characteristic temperature profiles of the battery under inert atmosphere during thermal runaway. The process can be divided into four distinct stages: heating, venting, thermal runaway, and cooling.
- Stage I (Heating): The battery temperature gradually increases as the heating plate is activated.
- Stage II (Venting): The battery’s vent valve opens at around 904 seconds, releasing pressure and causing a slight temperature drop.
- Stage III (Thermal Runaway): Internal reactions intensify, leading to a sharp rise in temperature and eventual voltage drop to zero.
- Stage IV (Cooling): The battery cools down as the heat source is removed.
4.2 Ambient Temperature
Ambient temperature changes during thermal runaway. The peak ambient temperature occurs near the battery’s left side, highlighting the need for adequate ventilation and thermal management.
4.3 Gas Production and Composition
Gas production under inert and air atmospheres was quantified using the ideal gas law. Total gas production and exhaust rates are presented, while the gas composition .
Under air atmosphere, hydrogen production decreased while methane and ethylene production increased due to enhanced chemical reactions. The overall gas production was 8.2% higher in air compared to inert atmosphere.
4.4 Flammability Limits
The flammability limits of the produced gases were calculated using Le Chatelier’s mixture law (Equation 6). Both atmospheres resulted in gas mixtures with high flammability limits, emphasizing the potential hazard.
4.5 Comparison of Test Atmospheres
Table 2: Comparison of Feature Temperatures
| Test Atmosphere | Open Valve Temp. (°C) | Thermal Runaway Start (°C) | Peak Temp. (°C) | Max. Heating Rate (°C/s) |
|---|---|---|---|---|
| Inert | 117.5 | 99.5 | 218.0 | 3.9 |
| Air | 122.3 | 102.3 | 256.3 | 4.1 |
Table 3: Comparison of Feature Times
| Test Atmosphere | Open Valve Time (s) | Thermal Runaway Duration (s) |
|---|---|---|
| Inert | 904 | 460 |
| Air | 888 | 524 |
The presence of air significantly increased the peak temperature, thermal runaway duration, and gas production, underscoring the importance of controlling test atmospheres in safety evaluations.
5. Conclusion
This study comprehensively analyzed the thermal runaway behavior of high-capacity LiFePO4 batteries under inert and air atmospheres. The results reveal that the air atmosphere significantly promotes thermal runaway, leading to higher temperatures, longer durations, and increased gas production. The gas compositions in both atmospheres exhibit high flammability limits, emphasizing the need for rigorous safety measures.
