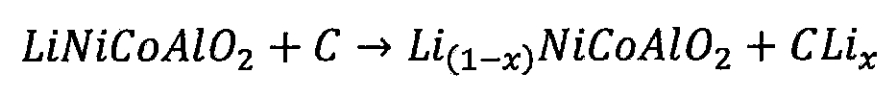1. Structure of lithium-ion batteries
Lithium ion batteries come in various shapes, with cylindrical and square being their most common forms. Lithium ion batteries are mainly composed of anodes, cathodes, electrolytes, and separators. Common lithium-ion batteries have a multi-layer structure inside. After arranging the positive electrode, separator, negative electrode, etc. of lithium-ion batteries in order, they are wound into a cylindrical shape or stacked into a square shape. After completion of winding or stacking, Then weld all the positive electrodes of the lithium-ion battery together to form the positive electrode, and lead them out to form the positive electrode column. All the negative electrodes are also welded together to form the negative electrode, and lead them out to form the negative electrode column. Figure 1 shows the internal structure of a cylindrical lithium-ion battery, with an aluminum sheet coated with cathode material as the positive electrode and a copper sheet coated with anode material as the negative electrode.
In lithium-ion batteries, the anode serves as a storage medium for lithium ions, and graphite, carbon black, lithium titanate, silicon, and graphene are all ideal anode materials. In current commercial lithium-ion batteries, the most common method is to coat graphite on copper foil as the anode, because graphite has a loose layered bonding structure, which can easily achieve reversible insertion of lithium ions into graphite.
The function of the cathode is to provide active lithium ions. Due to the presence of a valence electron in the outer layer of lithium, which has strong reactivity and is very unstable, stable metal lithium oxide is usually coated on aluminum foil as the cathode in commercial applications. Common metal lithium oxides include lithium nickel cobalt aluminate (LiNiCoA102, NCA), lithium nickel cobalt manganese oxide (LiNixMnyCoz02, NMC), lithium cobalt oxide (UC0O2, LCO) Lithium manganese oxide (LiMn204, LMO) and lithium iron phosphate (LiFeP04, LFP), etc. Different cathode materials have different properties, among which nickel cobalt lithium aluminate material has high energy density, but its structure is unstable, high temperature resistance is poor, and the production process requires high requirements and high cost; The energy density of lithium nickel manganese cobalt oxide materials is generally slightly lower than that of nickel cobalt aluminum materials, but their thermal stability will be better. Of course, one of the current research hotspots is the rich nickel nickel manganese cobalt lithium-ion battery, which improves energy density while its thermal stability is much worse than other batteries; Lithium cobalt oxide material has a stable structure and high specific energy, but its safety is poor and its price is high; Lithium manganese oxide material has low temperature resistance, good rate performance, good safety, and low price, but its chemical properties are unstable and its cycling performance is poor; Lithium iron phosphate material has high thermal stability, good safety, and good cycling performance, but low energy density.
Electrolytes are distributed on both sides of the separator, and their main function is to allow lithium ions to pass while preventing electrons from passing during the charging and discharging of lithium-ion batteries, in order to achieve the conduction of current between the positive and negative electrodes. Therefore, the electrolyte must be a good ionic conductor and electronic insulator. Electrolytes are typically composed of a mixture of organic carbonate solvents (such as ethyl carbonate or diethyl carbonate) and lithium ion salts (such as lithium hexafluorophosphate LiPF6, lithium perchlorate LiCl04, lithium hexafluoroarsenate monohydrate LiAsF6, lithium trifluoride LiCF3S03, and lithium tetrafluoroborate LiBF4).
The separator is located between the anode and cathode, usually made of plastic or polymer materials. Its main function is to separate the positive and negative electrodes of lithium-ion batteries, preventing short circuits caused by contact between the two poles. The diaphragm material is non-conductive, but it allows electrolyte ions to pass through. Different types of lithium-ion batteries use different separators. In addition, the performance of the separator also determines the interface structure, internal resistance and other parameters of lithium-ion batteries, which directly affect the capacity, cycling, and safety of lithium-ion batteries.
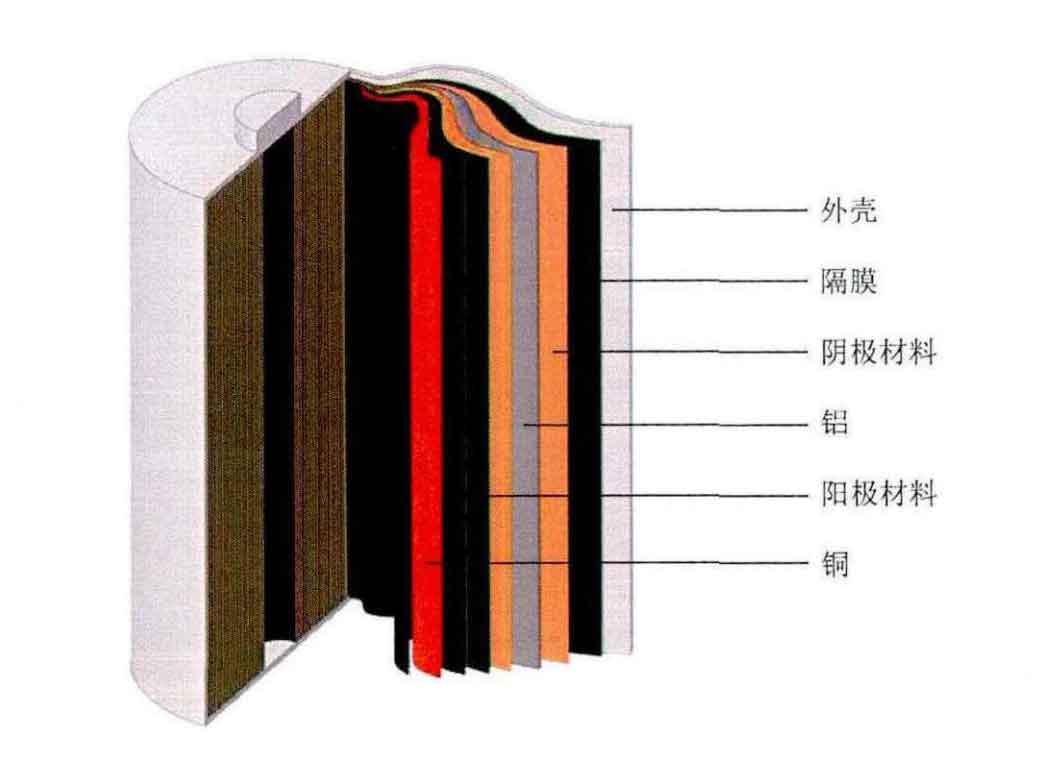
2. Working principle of lithium-ion batteries
The reactants for electrochemical reactions in lithium-ion batteries are anode materials and cathode materials, both of which are compounds containing lithium atoms, and these two electrodes allow lithium ions to enter and exit their structures through insertion (insertion) or extraction (extraction) processes, respectively. When a lithium-ion battery is discharged, the anode undergoes an oxidation half reaction, producing positively charged lithium ions and negatively charged electrons. The lithium ions pass through the electrolyte, and the electrons pass through the external circuit. Then, in the reduction half reaction, they recombine at the cathode (along with the cathode material). During this process, the electrolyte and external circuits provide conductive media for lithium ions and electrons respectively, but do not participate in electrochemical reactions. During discharge, electrons flow from the negative electrode (anode) to the positive electrode (cathode) through an external circuit. The reaction during the discharge process reduces the chemical potential of lithium-ion batteries, so discharge transfers energy from the lithium-ion battery to the place where the current dissipates energy (mainly in external circuits). When a lithium-ion battery is charged, these reactions and transfers are in the opposite direction, with electrons moving from the positive electrode to the negative electrode through an external circuit. In order to charge lithium-ion batteries, external circuits must provide electrical energy, which is then stored as chemical energy in the lithium-ion battery. Due to the back and forth oscillation of lithium ions between two electrodes, this type of lithium-ion battery is also known as a “rocking chair battery” or “rocking battery”. Figure 2 is a schematic diagram of the working principle of lithium-ion batteries.
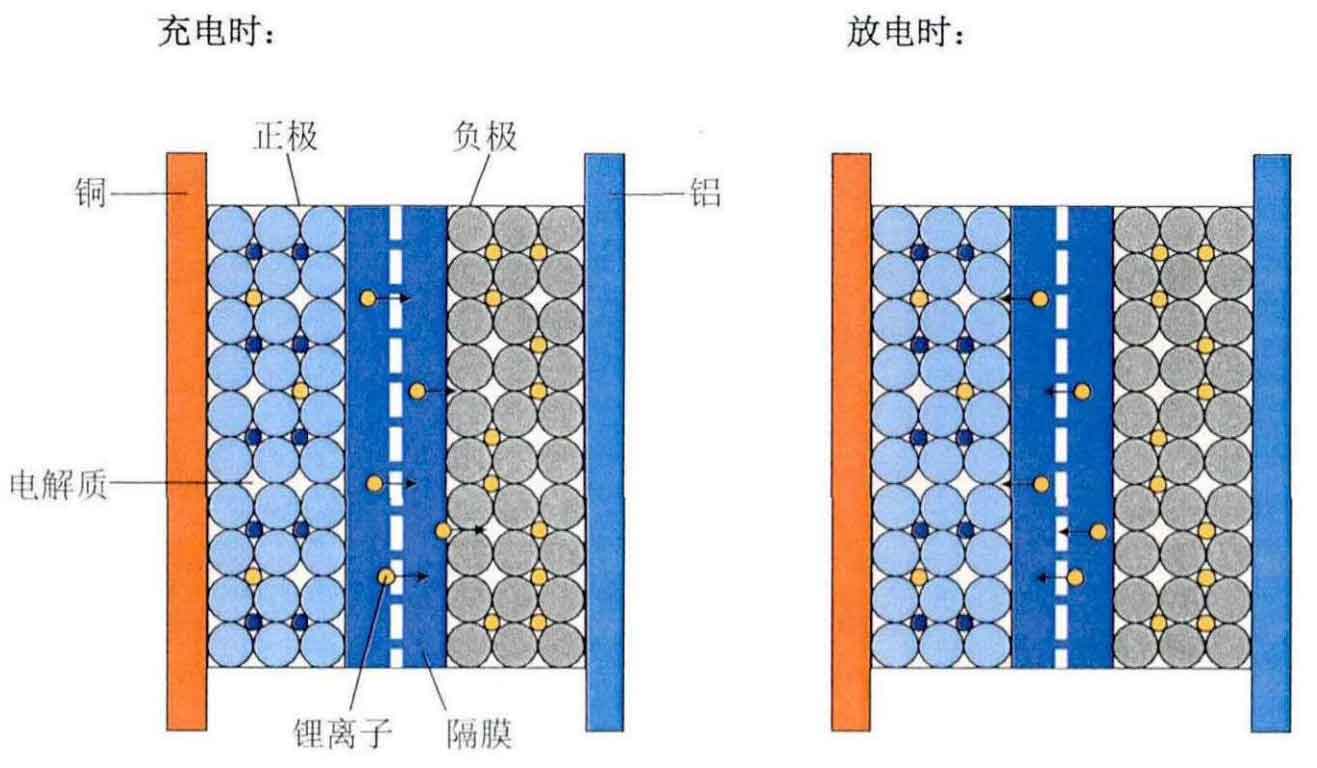
This article selects three types of lithium-ion batteries, namely nickel manganese cobalt, lithium cobalt oxide, and nickel cobalt aluminum, as the research objects. The chemical reactions that occur during the charging process are as follows (reverse reaction during discharge):
(I) Nickel cobalt battery
Lithium ion battery cathode:
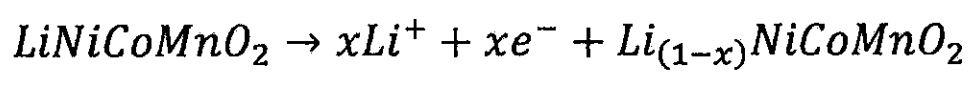
Lithium ion battery negative electrode:
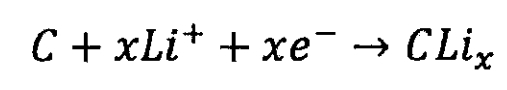
Total reaction of lithium-ion batteries:
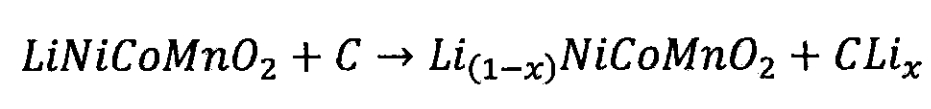
(2) Lithium cobalt oxide battery
Lithium ion battery cathode:
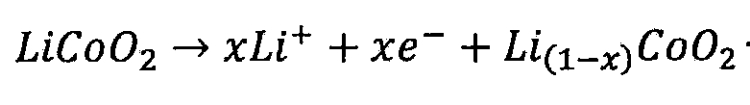
Lithium ion battery negative electrode:
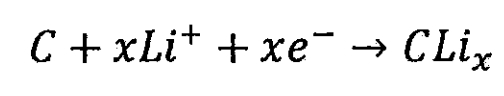
Total reaction of lithium-ion batteries:
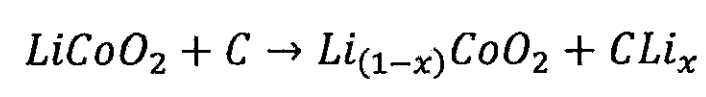
(3) Nickel cobalt aluminum battery
Lithium ion battery cathode:
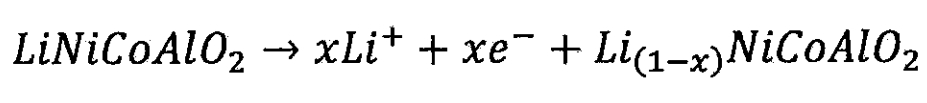
Lithium ion battery negative electrode:
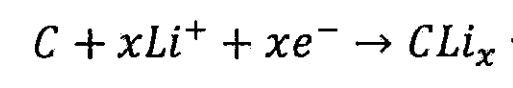
Total reaction of lithium-ion batteries: