Lithium ion batteries are currently widely used in the field of energy storage, and the frequent occurrence of fire and explosion accidents in energy storage plants has aroused great concern for the safety of electrochemical energy storage plants. Lithium ion batteries are the energy carriers for energy storage in power plants, and their electrode system components have high thermal hazard. After being packaged into batteries, their thermal hazard increases. In April 2021, an explosion occurred at the energy storage power station in Fengtai District, Beijing, resulting in the death of two firefighters, raising public concerns about the application prospects of the energy storage power station. The fire and explosion accidents that have occurred in energy storage power plants in recent years are shown in Table 1.
| Serial number | Date | Accident description | Possible causes of accidents |
| 1 | 2022.02.13 | Approximately 10 battery racks have been melted in the Moss Landing energy storage power plant project in California | Reason unknown |
| 2 | 2022.01.17 | A fire has occurred in the energy storage system of a solar power plant in Gyeongsangbuk Shingu, Yoshicheng, South Korea | Reason unknown |
| 3 | 2022.01.12 | A fire broke out in the battery storage building of SK Energy Company in South Ulsan District, South Korea | Battery overcharge |
| 4 | 2020.07.30 | Fire at Megapack, the largest energy storage plant in Tesla, Victoria, Australia | Unknown reason during testing |
| 5 | 2021.07.19 | Battery fire at Grand Ridge energy storage plant in Illinois, USA | Reason unknown |
| 6 | 2021.04.16 | A fire and explosion occurred at the lithium iron phosphate energy storage power plant in Jimei Dahongmen Shopping Mall, South Fourth Ring Road, Fengtai District, Beijing | Battery overcharging leads to thermal runaway |
| 7 | 2020.07.30 | Fire at Megapack, the largest energy storage plant in Tesla, Victoria, Australia | Unknown reason during testing |
| 8 | 2019.05 | A fire occurred in a container of a user side energy storage power plant in Beijing | During operation and maintenance |
| 9 | 2010.01.14 | Fire Occurred during Charging of the 5.22 MW Solar Energy Project in Quannanguan Island, South Korea | Single overcharging leads to thermal runaway |
| 10 | 2018.10.18 | Fire occurs during inspection and maintenance of energy storage containers for the 17.7 MW frequency modulation project in Gyeonggi do, South Korea | Thermal runaway of battery cells |
| 11 | 2018.08.03 | A fire broke out at a user side lithium iron phosphate energy storage power plant in Yangzhong, Jiangsu, resulting in the overall burning of an energy storage container | Caused by thermal runaway expansion after a battery caught fire |
| 12 | 2017.12.22 | A fire occurred in the No. 2 energy storage container of a 9 MW frequency regulation project in a power plant in Shanxi, accompanied by secondary disasters such as explosions | Short circuit in battery cell leads to thermal runaway |
| 13 | 2017.05 | A fire broke out during the rest of the ternary lithium battery energy storage unit in the frequency regulation project of an energy storage power station in Shanxi Province | Short circuit in battery cell leads to thermal runaway |
| 14 | 2017.03.07 | A fire broke out in the energy storage system of a thermal power plant in Shanxi, which destroyed one lithium-ion battery energy storage unit, 416 lithium-ion battery packs, and 26 battery management systems | Thermal runaway caused by overcharging |
The fire and explosion accidents of lithium-ion batteries in energy storage power plants are mainly caused by the internal short circuit of the battery cells, which leads to the thermal runaway of the battery and ignites, further spreading to adjacent batteries, forming a large-scale fire. When the gas volume accumulates to a certain extent in a confined space, it will encounter an ignition source and explode again. Although lithium-ion batteries have the risk of self triggering internal short circuits leading to thermal runaway, the probability is very low, only one in a million. It is generally believed that thermal runaway is caused by external induced conditions such as thermal abuse, electrical abuse, and mechanical abuse. When thermal runaway occurs in lithium-ion batteries in energy storage power stations, thermal runaway spread between batteries will occur, further triggering large-scale battery combustion, as shown in Figure 1.
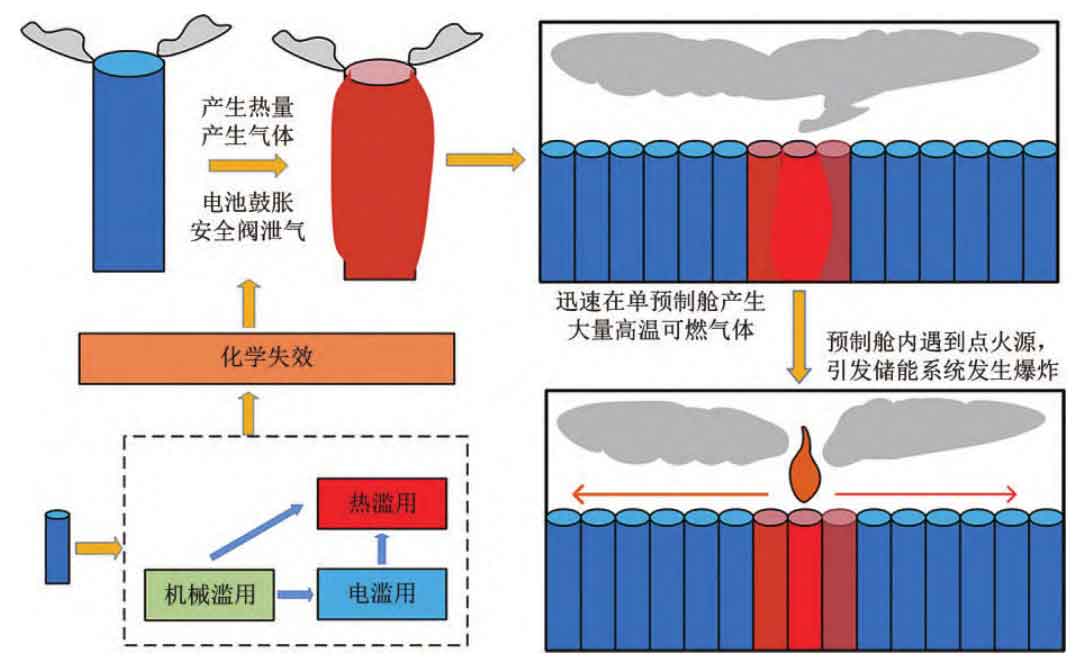
The process of lithium-ion batteries in energy storage power plants evolving from thermal runaway to fire and explosion can generally be divided into four stages: ① the battery releases heat under abusive conditions, producing flammable and toxic gases; ② Heat and combustible gases form a significant pressure in the enclosed space of the battery casing, causing leakage after opening the safety valve; ③ High temperature gas leakage forms jet fire or a large amount of high-temperature flammable and toxic mixture through the safety valve; ④ The high-temperature mixture accumulates in a single prefabricated storage structure and eventually encounters an ignition source, causing an explosion. Therefore, in order to prevent fire and explosion accidents in energy storage power plants, it is necessary and crucial to propose prevention and control measures based on the evolution process of thermal runaway.
1. Thermal runaway characteristics and evolution process of lithium-ion batteries in energy storage power plants
At present, research on the thermal runaway characteristics and evolution process of lithium-ion battery monomers at home and abroad mainly focuses on four aspects, namely the internal reaction timing law, characteristic temperature law, thermal runaway gas production law, and internal short circuit mechanism of the battery under various abuse conditions.
1.1 Thermal runaway internal reaction timing pattern
Thermal runaway is an irreversible temperature rise phenomenon caused by the sum of various side reactions that occur at high rates. The reason for thermal runaway is the overlapping cross side reactions that occur at the same time and space under various abusive conditions. When the side reactions reach a certain level, the diaphragm collapses, causing a large amount of heat to be released immediately due to a short circuit in the battery, resulting in thermal runaway of the battery, as shown in Figure 2.
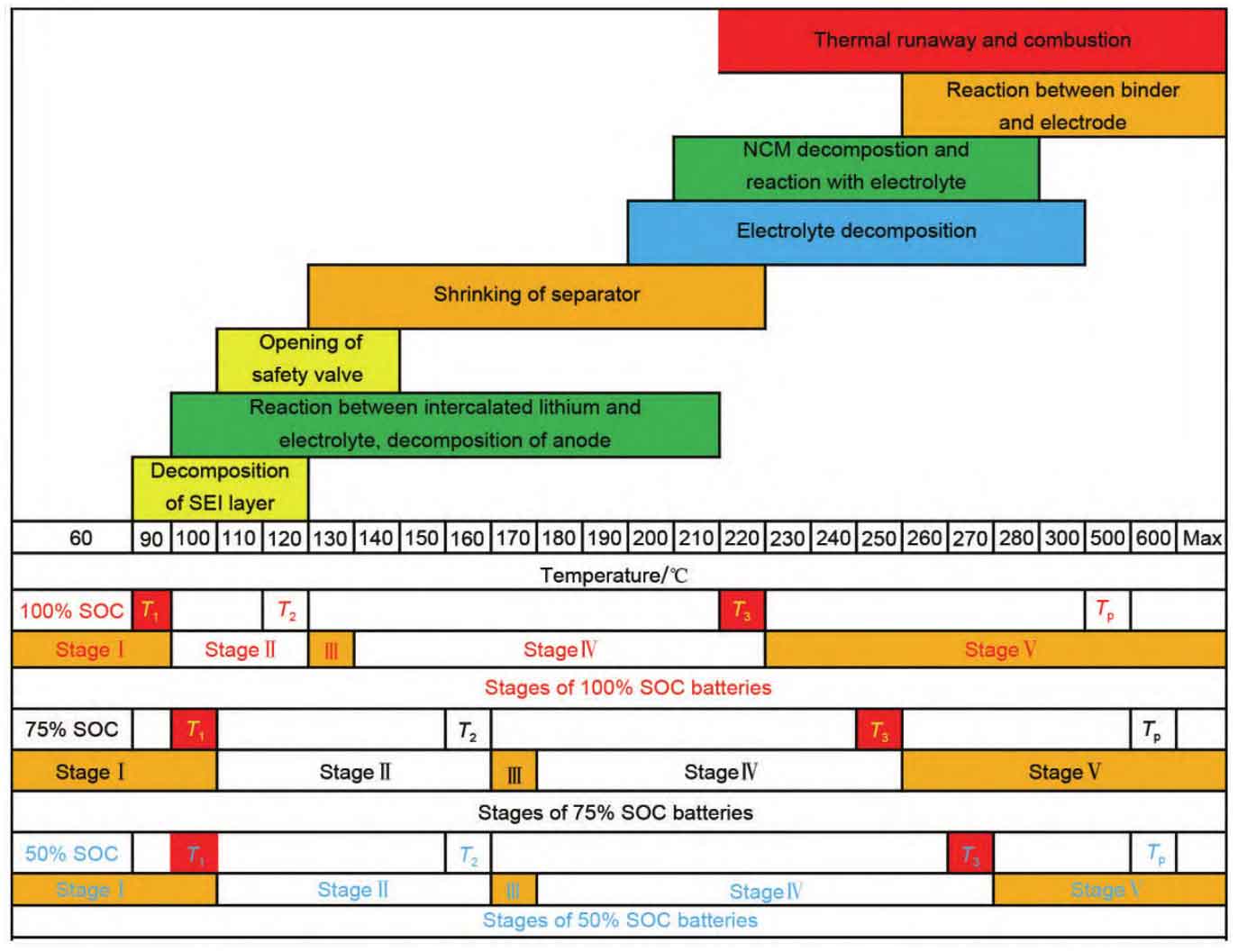
The internal side reactions of the battery are considered to be the key to generating heat accumulation inside the battery, so it is necessary to understand the reaction timing rules inside the battery. At present, it is widely believed that the following side reactions may occur internally from low to high temperatures after battery abuse: SEI film decomposition, thermal decomposition of positive electrode materials, reaction between lithium intercalated carbon and electrolyte, thermal decomposition of electrolyte, reaction between positive electrode materials and electrolyte, reaction between lithium intercalated carbon and binder, etc. Although these reactions have temperature dependent characteristics, they do not have a clear sequence of occurrence, It is more likely that overlapping and crossing occur at a certain temperature. When the heat accumulates to a certain extent, the diaphragm collapses, causing an internal short circuit, and then thermal runaway occurs, increasing the reaction rate to a certain extent, resulting in jet fire and deflagration. Hou et al. pointed out the pathway of oxygen evolution reaction leading to low thermal stability of batteries, confirming the importance of EC and anode in the process of thermal runaway evolution, which provides a way to cut off the chain reaction of thermal runaway to reduce the risk of thermal runaway. Chen et al. used electrolyte additives as “gas extinguishing agents” and “SEI&CEI improvers”, which can effectively suppress battery spray fire, proving the correctness of their approach.
1.2 Characteristic temperature pattern
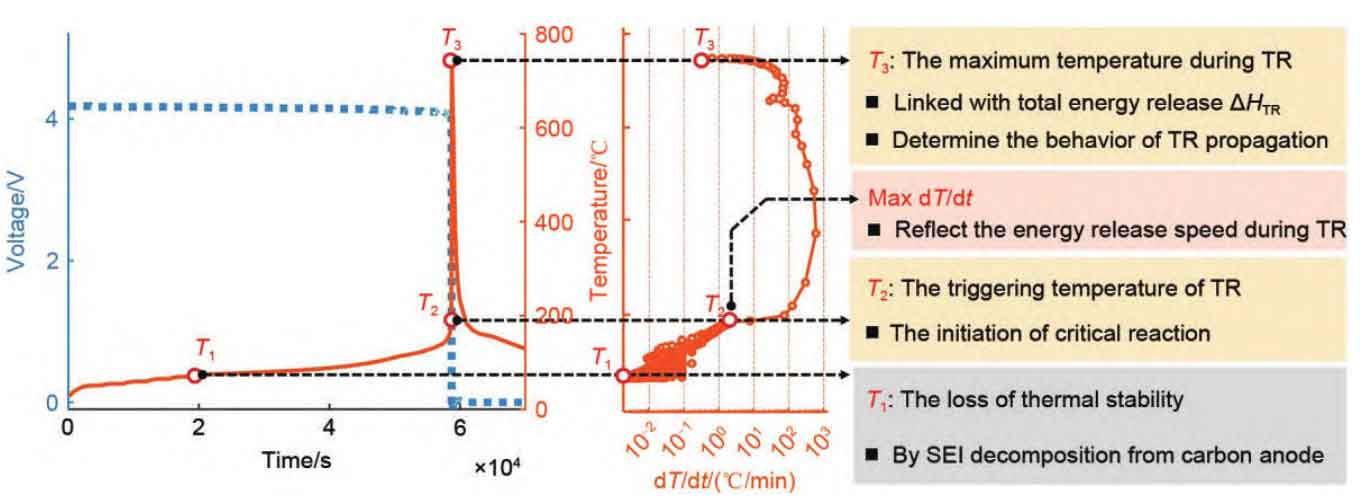
Feng et al. revealed the characteristic temperature law of thermal runaway and believed that there are three characteristic temperatures T1, T2, and T3 for thermal runaway, as shown in Figure 3. T1 is the starting temperature for self generated heat, from which the internal active substances begin to exhibit significant exothermic reactions. At this stage, each reaction overlaps and lasts for a long time; T2 is the triggering temperature for thermal runaway, which represents an internal short circuit inside the battery. Thermal runaway occurs at this time, and the temperature increases instantaneously. Gas is generated and rapidly accumulates, easily forming jet flames; T3 is the maximum temperature at which thermal runaway occurs, indicating the maximum temperature that the battery can reach when both thermoelectric and chemical energy is released. This temperature generally corresponds to the highest heat release rate and occurs almost simultaneously with the triggering temperature T2 of thermal runaway. Zhang et al., Liu et al., and Zhao et al. have all validated the correctness of this law in their studies on the use of different forms of abuse to cause uncontrolled contact fever. Based on this, thermal runaway can be divided into three periods: T1~T2 is the early stage of thermal runaway, T2~T3 is the period of thermal runaway occurrence, and T3 is the early stage of fire. The temperature law of thermal runaway characteristics can provide reference for thermal runaway prevention and control technologies and measures, that is, if the signal of thermal runaway evolution can be identified in the early stage of thermal runaway, the occurrence of fire accidents can be avoided.
1.3 Thermal runaway gas production law
The thermal runaway of the battery caused a fire event, and the internal side reactions of the battery not only contributed heat, but also released a large amount of flammable and toxic gases. Combustible gases rapidly generate in the enclosed space of the battery shell, forming a special phenomenon of lithium-ion battery fire, known as jet fire. Based on current measurements of the composition of thermal runaway gases, it has been found that common gases generated include CO, H2, CO2, CH4, C2H6, HF, electrolyte vapor, etc. Understanding the law of thermal runaway gas production helps to understand the ignition and explosion characteristics of batteries and provide prevention and control ideas. Furthermore, Mao et al. established a lumped model for the 18650 lithium battery, filling the knowledge gap regarding gas generation rate and jet velocity during thermal runaway processes. Li et al. [23] pointed out that breaking the boundary of the fire triangle can prevent the ignition of the thermal runaway gas based on the thermal runaway gas fire triangle. In addition, Zhang et al. evaluated the toxicity of gases, while Mier et al. provided a method for calculating the internal pressure accumulation in batteries, which improved understanding of thermal runaway gas production.
1.4 Internal short-circuit mechanism
Maleki et al. studied internal short circuits using experimental and thermal modeling methods. Santhanagopalan et al. simulated the possibility of internal short circuits in lithium-ion batteries, enhancing their understanding of internal short circuits. Ouyang et al. believe that the common process of thermal runaway under various abuse conditions is internal short circuit, and propose a method for detecting internal short circuit based on the consistency of batteries within the battery pack, which can help battery management systems achieve internal short circuit detection.
Current research indicates that internal short circuits are caused by diaphragm collapse, which is the direct cause of thermal runaway. The service conditions of lithium-ion batteries in energy storage stations are complex, which can easily lead to electrical abuse of the battery, causing lithium dendrites to form on the negative electrode of the battery, piercing the separator and causing internal short circuits. After an internal short circuit occurs in the battery, a large amount of heat is instantly released, causing the temperature of the battery to rapidly rise, leading to thermal runaway of the battery. The study of the mechanism of internal short circuits in batteries helps to understand the process of thermal runaway and predict internal short circuits in batteries.
In summary, during the process of thermal runaway evolution, the side reactions of lithium-ion batteries will generate both heat and gas. The increase in battery temperature is the result of heat accumulation, while the increase in battery internal pressure is the result of gas accumulation in the enclosed space of the battery shell. When heat and gas accumulate to a certain extent, the battery safety valve opens, spewing out a large amount of gas, and combustible gas and air quickly mix. The thermal runaway continues, and the chemical reaction rate rapidly accelerates, causing a sudden increase in the heating rate and gas generation rate. When the ignition conditions are met, the battery ignites and burns. Of course, it is also possible that the combustion caused by the ignition of combustible gases by electric sparks generated during the high-speed gas release process. For energy storage power plants, after local combustion, a large amount of high-temperature combustible and toxic mixed flue gas will undergo gas flow and migration. When combustible gas accumulates to a certain extent in a confined space, it will encounter an ignition source and cause gas explosion.
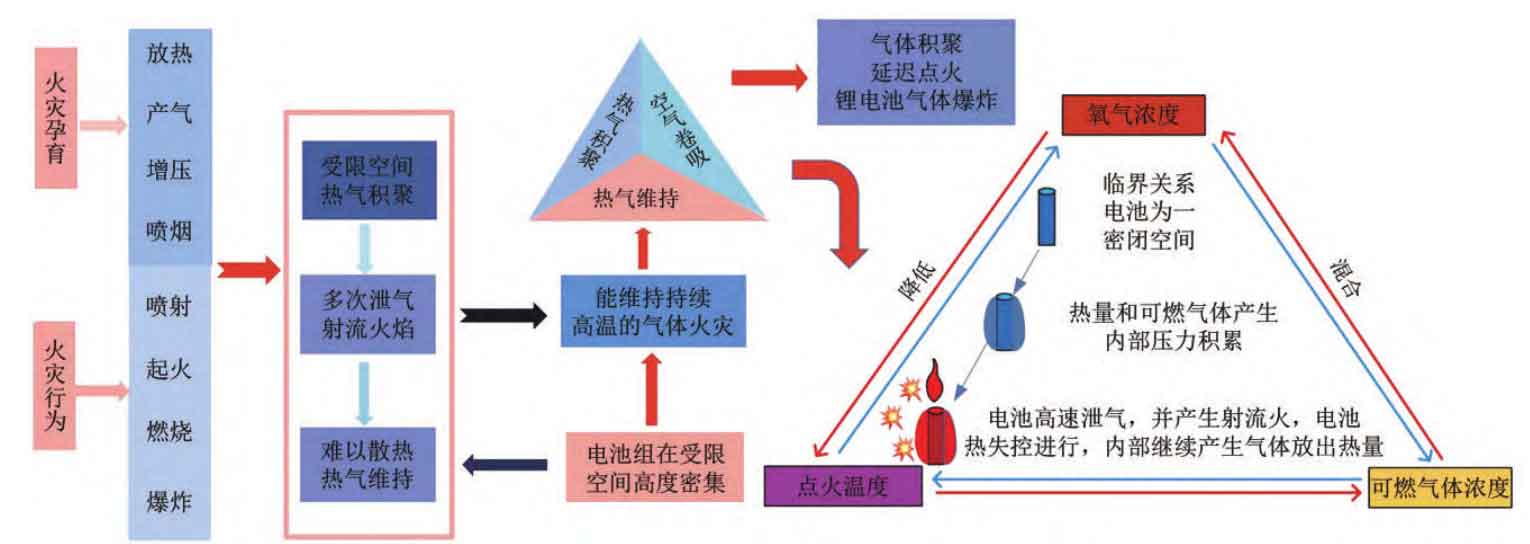
Based on this, the thermal runaway evolution process of lithium-ion batteries in energy storage power stations can be divided into six processes according to their thermal runaway characteristics: heat release, gas production, pressurization, smoke injection, ignition and combustion, and gas explosion, as shown in Figure 4. Understanding these six processes based on the characteristics of thermal runaway is the foundation for studying thermal runaway prevention and control technologies.
2.Thermal runaway monitoring and warning technology for lithium-ion energy storage systems
Based on the temperature characteristics of thermal runaway in lithium batteries mentioned above, the six processes of thermal runaway evolution are divided into three stages, namely the early stage of thermal runaway, the stage of thermal runaway occurrence, and the early stage of fire, as shown in Figure 5. The characteristic signals that occur during the six processes of thermal runaway evolution of batteries are electrical signals (voltage, current, resistance), temperature signals, gas signals, smoke signals, flame signals, etc. After forming an energy storage system, other signals such as wind, sound, vibration, strain, etc. may appear. Different technical means can identify characteristic signals in different stages of thermal runaway. In order to achieve the intrinsic safety of energy storage power plants, this article only introduces monitoring and warning technologies for the early and occurrence periods of thermal runaway.
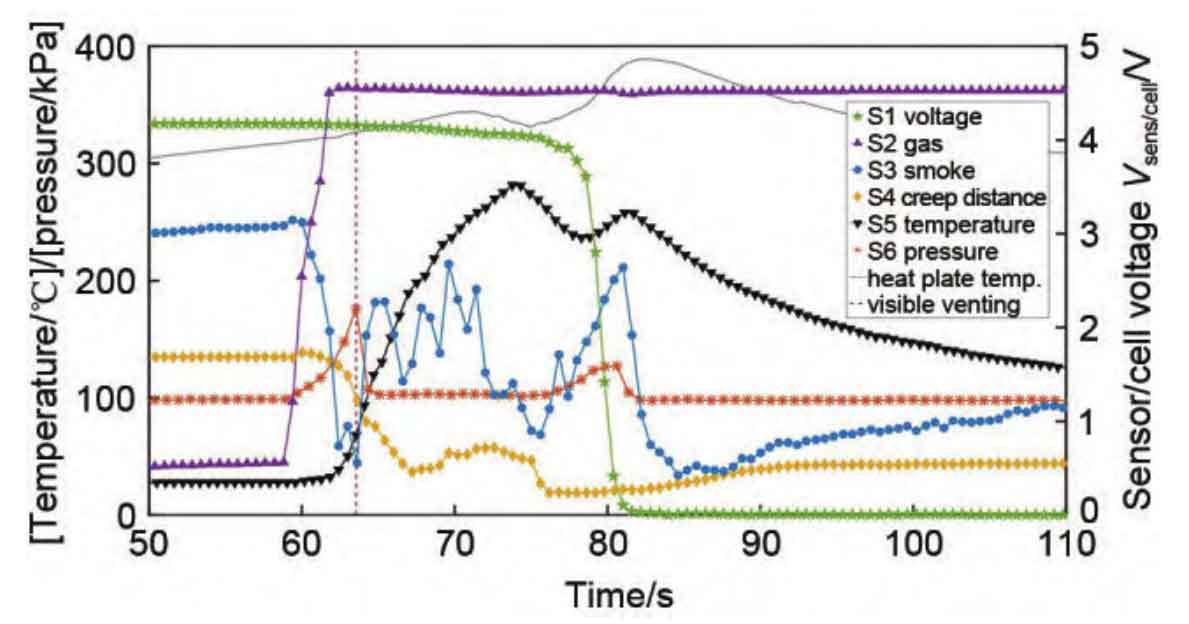
2.1 Temperature signal
Temperature is the most important signal in the process of thermal runaway, and battery thermal runaway is an irreversible process of temperature rise. This is an important parameter for determining the stage of battery thermal runaway, and temperature monitoring and warning are the most commonly used and basic methods.
Thermal runaway is an uncontrolled temperature rise process caused by many side reactions, which is a nonlinear process coupled with thermal electrical abuse, rather than a stable temperature rise process. Sun et al.’s research shows that there are differences in surface temperature and internal core temperature during normal operation of lithium batteries. Therefore, measuring the surface temperature alone cannot accurately determine whether the battery has experienced thermal runaway.
Wang et al. used infrared thermal imaging technology to obtain the temperature distribution of lithium batteries under different discharge rates and depths, which can effectively observe the temperature field changes of the battery over time and space. Rani et al.’s research demonstrated the applicability of this method.
Monitoring the temperature of lithium batteries based on fiber optic sensors is considered a high-precision measurement scheme. Alcock et al. used optical fiber sensors and K-type thermocouples to measure the surface temperature of batteries, and the results showed that the accuracy increased from ± 4.25 ℃ to+2.13 ℃. Yu et al. measured the temperature of lithium-ion batteries under different service conditions using distributed fiber optic sensors, and the results showed that the maximum temperature difference on the battery surface was 307% higher than that measured by traditional thermocouples.
In addition, Dong et al. found that electrochemical impedance spectroscopy has high sensitivity to internal abnormal temperature rise before the onset temperature of self generated heat in the mid frequency range, demonstrating the potential for early warning.
2.2 Gas signals
There are many reports on the phenomenon of thermal runaway and gas leakage, and the main components of the released gas are CO, CO2, HF, H2, and electrolyte vapor. The thermal runaway gas signal can be detected after the safety valve is opened. As the thermal runaway develops, the gas concentration increases and the types become more diverse.
Jin et al. reported a method for early warning of thermal runaway based on detecting H2. This method found that the formation of lithium dendrites can be detected based on H2 concentration detection, and even at a micrometer scale, it can be identified by detecting H2 concentration. Subsequently, overcharging experiments were conducted on the 8.8 kWh lithium iron phosphate module, indicating that H2 was first detected in six common gases: H2, CO, CO2, HCl, HF, and SO2. The detection time was 639 seconds earlier than smoke and 769 seconds earlier than fire.
2.3 Electrical signals
The electrical signal is an important signal that the battery management system constantly monitors, and the study of changes in electrical signals during thermal runaway is the key to early warning. Feng et al. conducted research on large capacity lithium-ion batteries using a large accelerated calorimeter, indicating a time delay of approximately 15 seconds between voltage drop and temperature rise. Meanwhile, through the small current pulse charging and discharging method, it was found that as the battery temperature increased, the resistance of the battery gradually increased. Ren et al. conducted in-depth research on this phenomenon and revealed the relationship between electrical signal changes caused by internal short circuits and temperature rise caused by thermal runaway.
The built-in voltage sensor of BMS can effectively monitor the terminal voltage of the battery. Once an abnormal signal is detected, an alarm can be issued quickly. The advantage of voltage monitoring is the ability to locate faulty batteries within the module. At the same time, the large number of batteries in energy storage stations requires the placement of more voltage sensors, resulting in higher computational costs.
At present, the monitoring and warning equipment for energy storage power plants mainly includes smoke alarms and temperature sensors. Existing research has shown that temperature based thermal runaway monitoring and warning methods cannot determine whether a battery has experienced thermal runaway based on surface temperature and predict internal temperature. Smoke detection technology only warns when thermal runaway occurs to a certain extent, and there is already a trend of fire occurring. VOC gas detection cannot distinguish whether the gas is a liquid leakage fault or a thermal runaway gas emission.
In summary, there is not much research on new methods for early warning of thermal runaway, and signal processing, cost, and engineering layout are also major challenges. Relying solely on a single parameter warning keeps the false alarm rate high. In the future, it is necessary to develop multi-parameter coupling warning technology to accurately identify the early stages of thermal runaway.
3. Thermal runaway suppression, fire extinguishing, and explosion suppression technology for lithium-ion energy storage systems
The lithium-ion battery clusters in energy storage power stations are tightly arranged in a single prefabricated storage room, and the batteries inside the clusters are highly dense, which can easily lead to thermal runaway expansion and spread. At this time, it is difficult to dissipate heat, and heat and combustible gases will gradually accumulate; If combustible gas diffuses and migrates and accumulates in a confined space, it is easy to explode after delayed ignition. Therefore, battery burning fires are gas fires that can maintain high temperatures. From the perspective of battery chemistry system and thermal runaway self generation characteristics, battery fires are caused by the self reaction of energetic materials with hot gas. It is crucial to take targeted prevention and control measures based on the three stages and six processes of thermal runaway evolution mentioned above.
3.1 Thermal runaway suppression technology
The existing thermal runaway suppression technologies mainly focus on cooling and blocking.
3.1.1 Thermal runaway cooling suppression technology
In terms of cooling methods, Liu et al. studied the suppression of thermal runaway of 3.7 V, 2.6 Ah NCM (1:1:1) battery cells under different SOC by water mist. Research has found that the occurrence of thermal runaway under continuous heating is unstoppable, but the surface temperature during thermal runaway can be reduced by spraying fine water mist. For high SOC, it is difficult for water mist to suppress thermal runaway, and the temperature has decreased by 20 ℃. For low SOC, the surface temperature decreased by at least 83.8 ℃, indicating that water mist has a stronger cooling ability for thermal runaway of low SOC batteries. For the module, it defines a cooling coefficient to determine the cooling effect of the fine water mist, and believes that when the surface temperature of the battery drops below 100 ℃, it can successfully prevent thermal runaway.
The batteries in energy storage power stations are generally connected in series and parallel, and the connection method has a significant impact on the propagation of thermal runaway. Liu et al. studied the effect of parallel connection on the thermal runaway propagation and active cooling of fine water mist in lithium-ion batteries. The experiment found that batteries connected in parallel exhibit lower initial temperature of thermal runaway, which leads to a decrease in the critical temperature node of water mist action. When the critical temperature drops below 100 ℃, the cooling process mainly relies on the heat absorption of water, which greatly reduces the control effect.
Huang et al. studied the cooling and suppression effects of liquid nitrogen on thermal runaway of 4.2 V, 2200 mAh LCO batteries. The results indicate that applying liquid nitrogen in the early stages of thermal runaway can successfully prevent the occurrence of thermal runaway. As the surface temperature of the battery increases, the inhibitory effect of liquid nitrogen on the battery weakens, but spraying 29.3 g of liquid nitrogen in 80 seconds will study the surface temperature of 9.24 Wh battery. Stop heating and apply fire extinguishing agent when the battery pressure relief valve is open. Experiments have shown that all extinguishing media can suppress the combustion of batteries. Carbon dioxide and HFC-227ea still exhibit flames during the release process, while fine water mist has no flame, indicating that extinguishing media with strong cooling ability have good suppression effects on lithium battery fires.
Huang et al. studied the cooling and suppression effects of liquid nitrogen on thermal runaway of 4.2 V, 2200 mAh LCO batteries. The results indicate that applying liquid nitrogen in the early stages of thermal runaway can successfully prevent the occurrence of thermal runaway. As the surface temperature of the battery increases, the inhibitory effect of liquid nitrogen on the battery weakens. However, spraying 29.3 g of liquid nitrogen in 80 seconds reduces the surface temperature of the 9.24 Wh battery from 700 ℃ to 100 ℃, demonstrating high cooling capacity. Due to the complex engineering layout of liquid nitrogen, its scale application is limited.
3.1.2 Thermal runaway blocking and suppression technology
In terms of barrier technology, Yuan et al. studied the effects of four gap materials, namely air, aluminum plate, graphite composite plate, and aluminum filling, on the propagation of thermal runaway. The study showed that graphite composite plate and aluminum filling can effectively suppress the propagation of thermal runaway. Niu et al. [56] studied the effect of low thermal conductivity and flame retardant composite phase change materials on suppressing thermal runaway propagation in square lithium batteries. The results indicate that the thermal runaway propagation of lithium battery packs with added barrier materials is suppressed. The research by Wen et al. [57] also indicates that the thermal runaway barrier technology of composite phase change materials can effectively suppress the propagation of thermal runaway and limit fire loads, which is of great significance for fire prevention and control.
3.2 Lithium battery fire extinguishing technology
The focus of research on lithium-ion battery fire extinguishing technology is mainly on the development and utilization of fire extinguishing media. Figure 6 shows the effectiveness and fire extinguishing strategies of common lithium-ion battery fire extinguishing media. Xu et al. [58] conducted experimental research on the suppression of lithium battery fires using three fire extinguishing agents: carbon dioxide, HFC-227ea, and fine water mist. Stop heating and apply fire extinguishing agent when the battery pressure relief valve is open. Experiments have shown that all extinguishing media can suppress the combustion of batteries. Carbon dioxide and HFC-227ea still exhibit flames during the release process, while fine water mist has no flame, indicating that extinguishing media with strong cooling ability have good suppression effects on lithium battery fires.
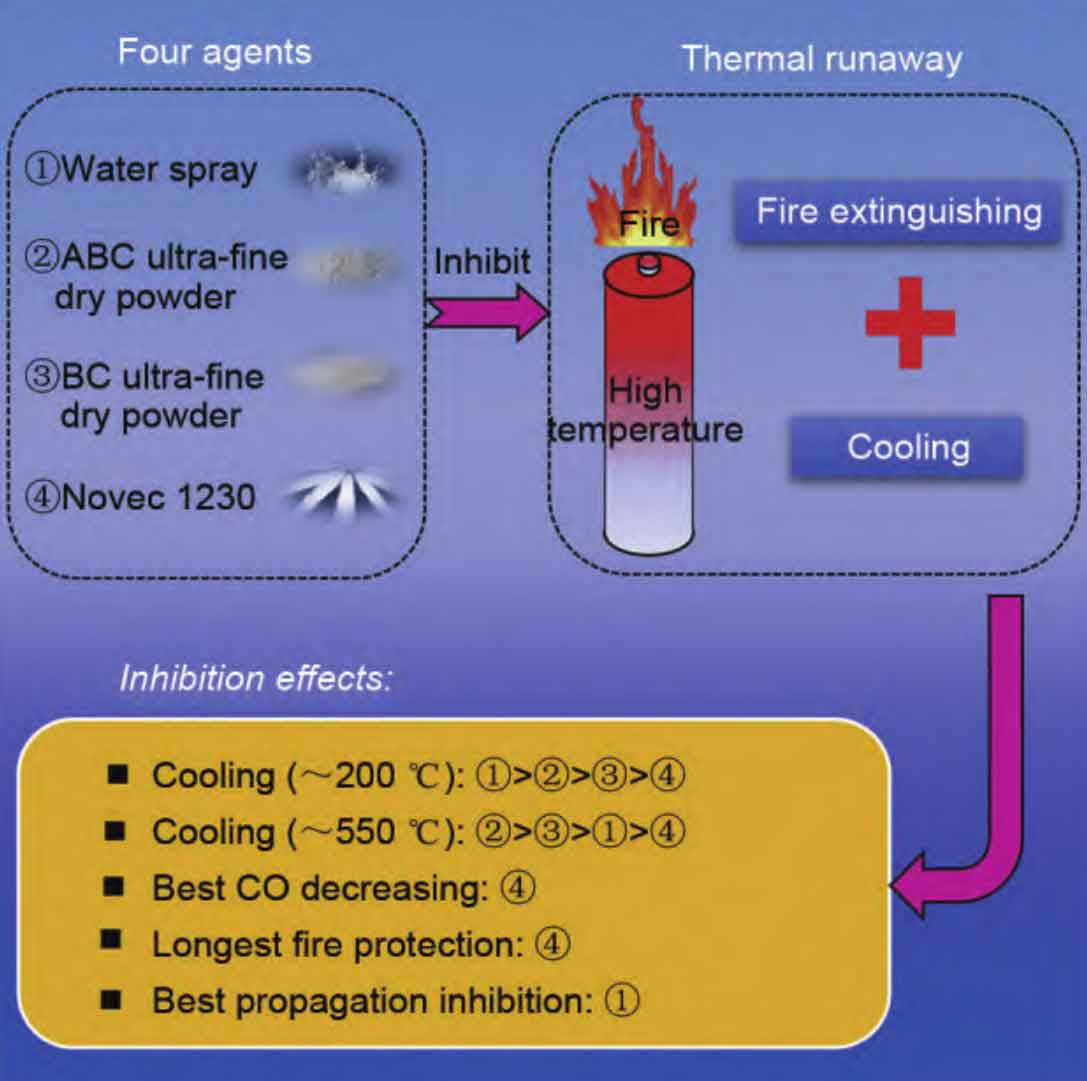
Liu et al. developed an integrated firefighting technology for fire suppression and rapid cooling. First, use perfluorohexaenone to extinguish the open flame of the battery, and then use fine water mist to cool it. The battery did not experience re ignition, while batteries that did not continue to cool with fine water mist experienced re ignition. The effectiveness of this secondary fire extinguishing technology lies in extinguishing gas fires first and then cooling them down. The experiment also proved that lithium-ion battery fires are gas fires that can maintain sustained high temperatures. Therefore, attention should be paid to the development of efficient gas extinguishing agents and continuous cooling agents as two extinguishing media.
At present, the research on fire extinguishing media mainly focuses on carbon dioxide, dry powder, foam, aerosol, heptafluoropropane, perfluorohexanone, water mist and other fire extinguishing agents. Previous studies have shown that compared to traditional energy fires, energy storage plant fires are often uncontrollable once they occur, and can only be passively extinguished and cooled by water spraying. This process targets the entire energy storage plant, causing all batteries to fail and become unusable. The batteries of energy storage power stations are highly concentrated in a single prefabricated compartment, making it impossible for fire extinguishing agents to enter the interior of the battery shell and directly act on the electrode material. Thermal runaway is still gestating, occurring, and expanding, making it highly susceptible to recurrence
Burning. Therefore, accurately identifying the characteristic signals of thermal runaway in the early stage of thermal runaway and taking timely measures to suppress thermal runaway are relatively safe technical means, which can successfully suppress the evolution of lithium batteries in energy storage stations from single thermal runaway to large-scale fire accidents.
3.3 Explosion suppression technology for energy storage power plants
After a short circuit in the battery cell of an energy storage power station causes a fire and combustion, due to the highly dense arrangement of the batteries, it is easy to form a phenomenon of heat runaway transmission. At this point, the adjacent area battery is in the process of thermal runaway evolution, which will generate a large amount of combustible gas and accumulate in the confined space, which can cause an explosion under certain conditions.
The explosion of an energy storage power station is a gas explosion, which generally takes two forms based on the type of battery: delayed ignition explosion and supplementary oxygen explosion. Delayed ignition explosion refers to the movement of a large amount of combustible smoke into a confined space, reaching the explosion limit and encountering an ignition source before explosion occurs; Supplementary oxygen explosion refers to the accumulation of heat and combustible gases in a confined space. When doors and windows are opened, oxygen is introduced and an explosion occurs. For lithium iron phosphate energy storage power plants, delayed ignition explosions are more likely to occur; For ternary lithium energy storage power plants, oxygen replenishment explosions are more likely to occur. The core of explosion suppression technology for energy storage power plants is to prevent the accumulation of combustible gases in confined spaces from reaching the explosion limit. Therefore, it is urgent to study the disposal measures and delayed ignition control schemes for the accumulation of energy storage electricity and combustible gas. Active ventilation measures are necessary and crucial, which require research on the gas generation rate, total gas production, and gas composition of large-scale battery arrays. Zhang et al. studied the composition and explosion limit of thermal runaway gases under different SOC conditions, while Chen et al. provided theoretical support for the rationality of dilution inerting. In addition, the development of explosion suppressants is also a feasible solution. Zhu et al. studied the explosion suppression efficiency of fine water mist as an explosion suppressant when a large amount of combustible gas is generated.
4. Conclusion and Outlook
At present, research on the mechanism and evolution process of thermal runaway has been relatively in-depth, and there are still many problems to be solved in the monitoring, warning, and prevention and control technology of lithium-ion batteries in energy storage stations. This article reviews the thermal runaway characteristics, evolution process, and prevention and control technologies of lithium batteries in energy storage power plants, and draws the following conclusions.
(1) The thermal runaway evolution process of lithium-ion batteries in energy storage stations under external abuse conditions can be divided into three stages and six processes. The three stages are the early stage of thermal runaway, the period of thermal runaway occurrence, and the early stage of fire. The six processes are heat release, gas production, pressurization, smoke injection, ignition and combustion, and gas explosion. The entire evolutionary process is not independent of each stage, but rather involves overlapping and intersecting chemical reactions. Only by deeply understanding the thermal runaway characteristics and evolution process of lithium batteries can reliable and advanced monitoring, warning, suppression, fire extinguishing, and explosion suppression technologies be obtained.
(2) In terms of monitoring and early warning for energy storage power plants, electrical signals, temperature signals, and gas signals as a single monitoring signal have poor warning effects. In the future, it is necessary to build a multi-parameter coupled monitoring and warning technology for the entire process of thermal runaway based on electrical signals, with temperature and gas signals as the core, and smoke and flame signals as auxiliary. Based on the warning results, corresponding accident handling measures should be provided, such as early thermal management for thermal runaway, power outage cooling and suppression during the occurrence of thermal runaway, and fire extinguishing in the early stage of fire.
(3) In terms of thermal runaway suppression, fire extinguishing, and explosion suppression technology. During the period of thermal runaway, using barrier technology to limit the number of thermal runaway modules to a certain range, and then cooling them can effectively prevent fire accidents and achieve a safe response to thermal runaway in energy storage power plants. In the early stages of a fire, it is necessary to use extinguishing media or extinguishing technologies that can both extinguish gas fires and efficiently cool down to suppress energy storage plant fires based on the characteristics of lithium battery fires. At the same time, lithium batteries in energy storage power plants are prone to gas diffusion and accumulation in confined spaces after thermal runaway, resulting in delayed ignition and explosion. Based on this, effective ventilation dilution, inerting, and explosion suppression technologies can be developed.
