In recent years, the development of energy storage technologies has accelerated, with a particular focus on improving safety and performance. Conventional liquid lithium-ion batteries are prone to risks such as electrolyte leakage, combustion, and explosion when subjected to overcharge, over-discharge, or short circuits. In contrast, solid-state batteries offer significant advantages in safety that liquid lithium-ion batteries cannot match. This has driven extensive research into solid-state battery technologies, especially for high specific energy applications. However, the absence of standardized test methods for electrical performance parameters of high specific energy solid-state batteries poses a challenge. Existing test methods for liquid lithium-ion batteries are not directly applicable due to differences in material properties and operational mechanisms. Therefore, it is crucial to develop specific test methods that address the unique characteristics of high specific energy solid-state batteries, ensuring they meet the考核 requirements for engineering applications.
High specific energy solid-state batteries are designed to achieve a gravimetric energy density of up to 450 Wh/kg, which surpasses that of long-cycle solid-state batteries (typically around 350 Wh/kg) and conventional liquid lithium-ion batteries (up to 400 Wh/kg). This improvement is attributed to the use of solid electrolytes, which reduce weight and enhance energy storage capacity. Additionally, these batteries demonstrate robust performance across a wide temperature range. For instance, at -20°C, the discharge capacity retention is approximately 70% of that at room temperature (25°C), which is comparable to liquid lithium-ion batteries. However, at elevated temperatures, solid-state batteries excel; at 80°C, they maintain about 80% of their room temperature discharge capacity, whereas liquid lithium-ion batteries often experience severe side reactions, leading to potential thermal runaway, fire, or explosion above 55°C. These distinctions highlight the need for tailored test methods to evaluate key electrical parameters, such as gravimetric energy density, discharge capacity retention at 1C rate, cycle life, low-temperature discharge capacity, and high-temperature discharge capacity.
To address this gap, we conducted a series of experiments on typical high specific energy solid-state battery samples. The test samples were selected to represent common configurations used in practical applications, ensuring the relevance of our findings. All tests were performed under controlled environmental conditions, with temperature maintained at 25±3°C unless specified otherwise. The equipment included an ET-1000L-C2 high-low temperature test chamber, a BTS-5V200A16CH battery testing system, and a BT300 electronic balance. All instruments were calibrated to ensure accuracy, with temperature measurements within ±0.5°C, dimensional and mass measurements within ±0.1%, and voltage and current measurements within ±0.5%.
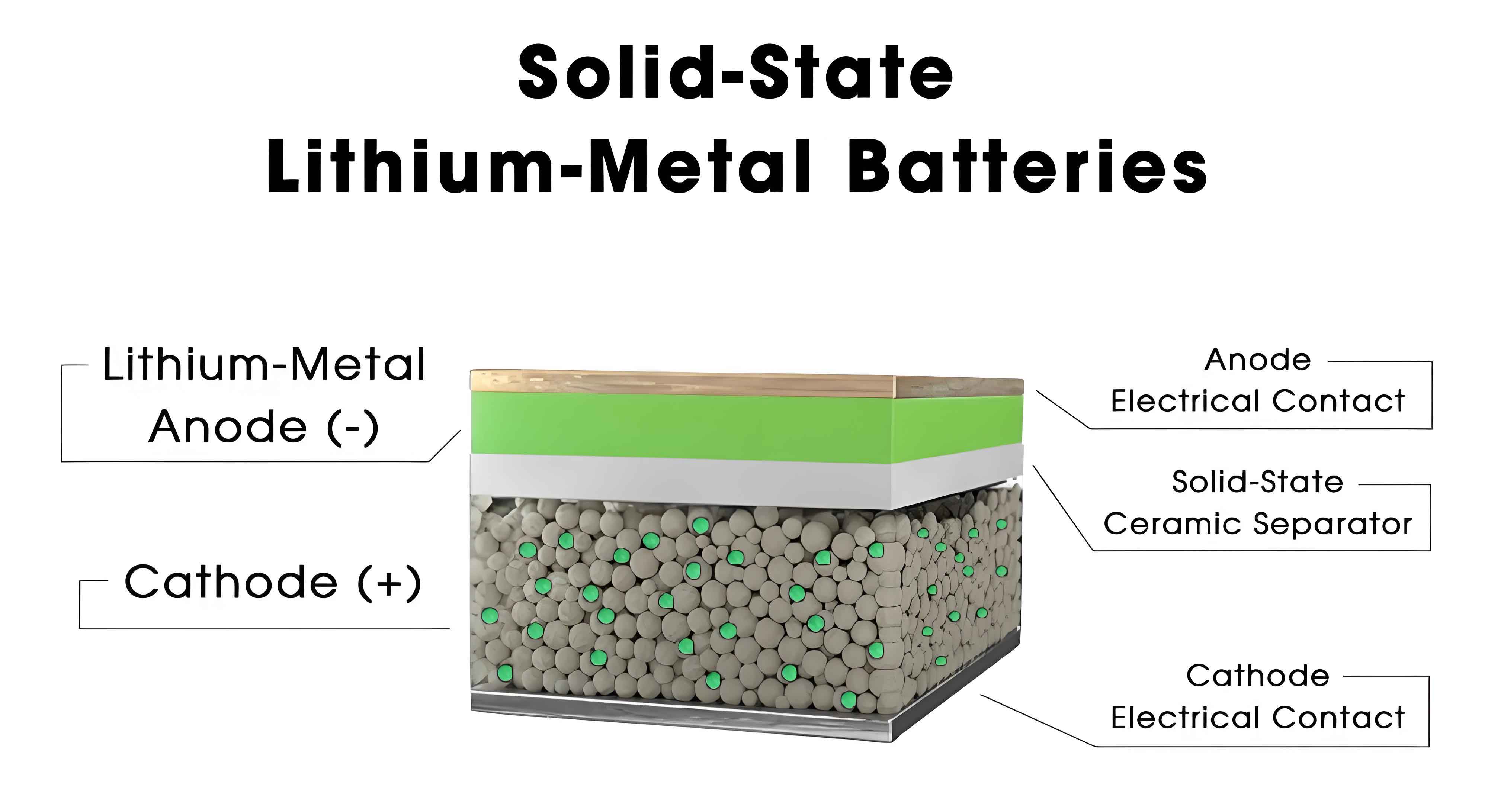
The gravimetric energy density test is a critical parameter for evaluating the energy storage efficiency of solid-state batteries. In this test, the battery was charged at a constant current of 0.1C to a voltage of 4.35V under room temperature conditions. After a resting period of 15 minutes, it was discharged at 0.1C to a cutoff voltage of 3.0V. The discharge capacity (in Ah) and energy (E in Wh) were recorded directly from the testing software. The gravimetric energy density (E_w) was calculated using the formula: $$E_w = \frac{E}{W}$$ where W is the weight of the battery in kilograms. For the sample tested, the discharge energy was 57.12 Wh (based on an average voltage of 3.912V and a capacity of 14.6 Ah), and the weight was 0.1259 kg, resulting in a gravimetric energy density of 453.6 Wh/kg. This meets the requirement of E_w ≥ 450 Wh/kg for high specific energy solid-state batteries. The results underscore the superiority of solid-state batteries in terms of energy density, which is essential for applications requiring lightweight and high-energy storage, such as electric vehicles and portable electronics.
Next, the 1C discharge capacity retention rate was evaluated to assess the battery’s performance under higher discharge rates. The battery was first charged at 0.2C to 4.35V and rested for 15 minutes. It was then discharged at 1C to 3.0V, and the discharge capacity was compared to the initial capacity measured at 0.2C discharge. The initial discharge capacity at 0.2C was 14.28 Ah, while the 1C discharge capacity was 13.62 Ah, yielding a retention rate of 95.4%. This exceeds the minimum requirement of 80%, indicating that solid-state batteries maintain high efficiency even under demanding discharge conditions. The high retention rate is attributed to the stable interface between the solid electrolyte and electrodes, which minimizes resistance and capacity loss. This property is crucial for applications involving rapid discharge, such as power tools and emergency backup systems.
Cycle life testing is vital for determining the longevity of solid-state batteries. The battery underwent repeated charge-discharge cycles at room temperature. Each cycle involved charging at 0.2C to 4.35V, resting for 15 minutes, and discharging at 0.5C to 3.0V. After 200 cycles, the discharge capacity was measured and compared to the initial capacity. The initial capacity was 14.5 Ah, and the capacity after 200 cycles was 11.75 Ah, resulting in a retention rate of approximately 81.03%. This satisfies the requirement of ≥80% capacity retention after 200 cycles. The gradual capacity fade observed is typical for solid-state batteries due to factors like electrode degradation and interfacial changes, but the results confirm their robustness for long-term use. To illustrate the cycle life data, the following table summarizes the capacity retention over selected cycles:
| Cycle Number | Discharge Capacity (Ah) | Capacity Retention (%) |
|---|---|---|
| 1 | 14.50 | 100.00 |
| 50 | 13.80 | 95.17 |
| 100 | 13.00 | 89.66 |
| 150 | 12.30 | 84.83 |
| 200 | 11.75 | 81.03 |
Low-temperature discharge capacity was tested to evaluate performance in cold environments. The battery was charged at 0.2C to 4.35V and rested for 15 minutes. It was then placed in a -20±2°C chamber for 8 hours before being discharged at 0.2C to 3.0V. The discharge capacity at low temperature was 11.48 Ah, compared to the room temperature capacity of 13.98 Ah, giving a retention rate of 82.12%. This exceeds the requirement of ≥70%, demonstrating that solid-state batteries can operate reliably in sub-zero conditions. The solid electrolyte’s inherent properties reduce the risk of freezing and maintain ion conductivity, which is a significant advantage over liquid electrolytes that can solidify or exhibit increased viscosity at low temperatures.
High-temperature discharge capacity was assessed to examine thermal stability. The battery was charged similarly and then placed in an 80±2°C chamber for 8 hours before discharge at 0.2C to 3.0V. The discharge capacity at high temperature was 14.5 Ah, compared to the room temperature capacity of 13.98 Ah, resulting in a retention rate of 103.7%. This surpasses the requirement of ≥80%, highlighting the exceptional thermal resilience of solid-state batteries. The solid electrolyte minimizes side reactions and decomposition at high temperatures, whereas liquid electrolytes in conventional batteries can lead to gas generation and thermal runaway. The performance can be modeled using the Arrhenius equation to describe the temperature dependence of capacity: $$C(T) = C_0 \cdot e^{-\frac{E_a}{RT}}$$ where C(T) is the capacity at temperature T, C_0 is the reference capacity, E_a is the activation energy, R is the gas constant, and T is the absolute temperature. For solid-state batteries, the low E_a values contribute to stable performance across temperatures.
Based on these experimental results, we have developed a standardized test method for the electrical performance parameters of high specific energy solid-state batteries. This method includes detailed procedures for gravimetric energy density, 1C discharge capacity retention, cycle life, low-temperature discharge capacity, and high-temperature discharge capacity. The environmental conditions for all tests are specified as temperature: 15°C to 35°C, relative humidity: 20% to 80%, and atmospheric pressure: 86 kPa to 106 kPa, unless otherwise stated. The charging protocol involves discharging the battery at 0.2C to 3.0V, resting for 30 minutes, and then charging at 0.2C to 4.35V with a 15-minute rest. For gravimetric energy density, the battery is discharged at 0.1C after charging, and E_w is calculated as described. The 1C discharge capacity retention test compares the discharge capacity at 1C to the initial capacity at 0.2C. Cycle life testing involves 200 cycles of charge and discharge at 0.5C, with capacity retention calculated. Low-temperature and high-temperature tests involve conditioning the battery at -20°C and 80°C, respectively, for 8 hours before discharge.
The requirements for each parameter are as follows: gravimetric energy density must be at least 450 Wh/kg; 1C discharge capacity retention must be no less than 80% of the initial capacity; after 200 cycles, the capacity retention should be ≥80%; low-temperature discharge capacity must be ≥70% of the room temperature capacity; and high-temperature discharge capacity must be ≥80% of the room temperature capacity. These criteria ensure that high specific energy solid-state batteries meet the demands of various applications, from consumer electronics to aerospace. The test method has been applied in the development of product specifications and testing reports for institutions, facilitating the adoption of solid-state battery technology. For instance, it has supported the evaluation of solid-state batteries in projects requiring high energy density and safety, such as unmanned aerial vehicles and renewable energy storage systems.
In conclusion, through systematic experimentation and analysis, we have established comprehensive test methods for the electrical performance parameters of high specific energy solid-state batteries. These methods address the unique properties of solid-state batteries, including their high gravimetric energy density, excellent rate capability, long cycle life, and superior performance at extreme temperatures. The incorporation of formulas, such as $$E_w = \frac{E}{W}$$ for energy density and the Arrhenius equation for temperature effects, along with tabulated data, provides a robust framework for evaluation. This work fills a critical gap in the standardization of test methods for solid-state batteries, enabling accurate assessment and comparison of products. As the adoption of solid-state batteries grows, these methods will play a vital role in ensuring reliability and safety, ultimately supporting advancements in energy storage technology. Future research could focus on optimizing these methods for emerging solid-state battery chemistries and scaling them for large-scale production.
