Abstract: This article focuses on the recycling of lithium-iron battery, specifically exploring the recovery process of valuable components from spent LiFePO4 battery. It examines various aspects such as the background of lithium-iron battery development, the importance of recycling, different recycling methods, and the kinetics of the leaching process. Through detailed research and analysis, it provides a comprehensive understanding of the lithium-iron battery recycling field and offers insights for future research and development directions.
1. Introduction
Lithium-iron battery, especially LiFePO4 battery, have gained significant popularity in recent years due to their excellent performance characteristics. These lithium-iron batteries are widely used in electric vehicles and energy storage systems. However, with the increasing number of battery applications, the issue of lithium-iron battery recycling has become crucial.
| Battery Application | Market Share | Growth Trend |
|---|---|---|
| Electric Vehicles | High | Increasing |
| Energy Storage Systems | Moderate | Steady Growth |
The recycling of lithium-iron battery not only helps in conserving valuable resources but also addresses environmental concerns associated with battery disposal.
2. The Development and Structure of Lithium-Iron Battery
Lithium-iron battery have evolved over time to meet the growing demands of various applications. The LiFePO4 battery, in particular, has a unique structure that contributes to its performance.
| Battery Component | Function | Material |
|---|---|---|
| Cathode | Determines battery capacity and voltage | LiFePO4 |
| Anode | Stores lithium ions during charging | Graphite |
| Electrolyte | Facilitates ion movement | Lithium salts in organic solvents |
| Separator | Prevents short circuits | Polymeric materials |
The electrochemical reactions within the LiFePO4 battery involve the intercalation and deintercalation of lithium ions between the cathode and anode.
3. The Importance of Recycling Lithium-Iron Battery
As the production and use of lithium-iron battery increase, the need for recycling becomes more evident.
| Recycling Benefit | Description |
|---|---|
| Resource Conservation | Recovers valuable metals like lithium and iron |
| Environmental Protection | Reduces the risk of toxic substances from battery disposal |
| Economic Viability | Creates a new revenue stream from recycled materials |
Failure to recycle these lithium-iron battery can lead to resource wastage and potential environmental hazards.
4. Recycling Methods for Lithium-Iron Battery
There are several methods available for recycling lithium-iron battery, each with its own advantages and limitations.
| Recycling Method | Advantages | Limitations |
|---|---|---|
| Direct Repair and Regeneration | Preserves original lattice structure, simple process, low cost | Difficulty in meeting new electrode material standards |
| Hydrometallurgical Process | Strong raw material applicability, flexible product options, good resource recovery | Complex process, requires multiple purification steps |
The hydrometallurgical process, in particular, involves several steps such as pretreatment, leaching of valuable metals, purification of the leachate, and product preparation.
5. The Leaching Process of Spent LiFePO4 Battery Electrodes
The leaching process is a crucial step in the hydrometallurgical recycling of LiFePO4 battery. It involves the extraction of valuable metals such as lithium and iron from the electrode powder.
| Leaching Parameter | Influence on Leaching |
|---|---|
| Sulfuric Acid Concentration | Affects the solubility of metals, higher concentration generally leads to higher leaching rates |
| Reaction Temperature | Increases reaction rate with higher temperature, but too high a temperature may cause evaporation and safety issues |
| Electrode Powder Particle Size | Smaller particle size provides larger surface area, enhancing leaching efficiency |
| Stirring Speed | Promotes mass transfer and improves leaching rate |
A detailed study was conducted to optimize these parameters and understand the leaching kinetics.
6. Kinetic Studies of the Leaching Process
The leaching kinetics of lithium and iron from spent LiFePO4 electrode powder was investigated using various models and techniques.
| Kinetic Model | Application in Leaching Process |
|---|---|
| Avrami Model | Successfully describes the leaching kinetics of multi-metal reactions in solid/liquid systems |
The study found that the leaching process is controlled by external diffusion, and the apparent activation energies for iron and lithium during the leaching process were determined to be 11.03 kJ/mol and 8.45 kJ/mol, respectively.
The leaching kinetic equations for iron and lithium can be expressed as follows:
For iron:
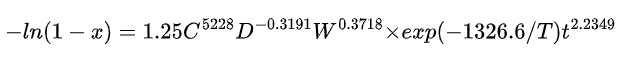
For lithium:
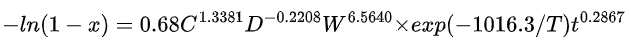
where x is the volume fraction of the leached substance, C is the acidity (mol/L), D is the particle size (mm), W is the rotation speed (r/min), and T is the absolute temperature (K).
7. Challenges and Future Directions in Lithium-Iron Battery Recycling
Despite the progress made in lithium-iron battery recycling, there are still several challenges that need to be addressed.
| Challenge | Description |
|---|---|
| Complex Battery Structures | Difficulties in disassembling and separating components |
| Purification of Leachate | Ensuring high purity of recovered metals |
| Cost-Effectiveness | Balancing the cost of recycling with the value of recovered materials |
Future research should focus on developing more efficient recycling methods, improving the purification processes, and reducing the overall cost of recycling.
In conclusion, the recycling of lithium-iron battery, especially LiFePO4 battery, is of great importance. Through continuous research and development, it is possible to overcome the existing challenges and achieve more sustainable battery recycling practices. This will not only contribute to resource conservation and environmental protection but also support the growth of lithium-iron battery industry.
