Four phenomena can be observed from the calendar aging test:
(1) At all test temperatures, the capacity decay of lithium-ion batteries with SOC of 0% is significantly lower than that with SOC of 50% and 100%. When the testing temperature is -40 ° C and -5 ° C, the calendar aging of lithium-ion batteries with a SOC of 50% is very close to that of batteries with a SOC of 100%. However, when the testing temperature is 50 ° C, the difference between the two is very significant.
This is because a high cathode potential leads to electrolyte oxidation and dissolution of transition metals in the cathode, while a low anode potential exacerbates electrolyte depletion at the anode. The decomposition of electrolytes consumes recyclable lithium and forms solid electrolyte interphase on the cathode and anode, respectively. The higher the temperature, the faster these reactions, while the higher the SOC, the higher the cathode potential, the lower the anode potential, and the faster the aging of lithium-ion batteries.
(2) When the testing temperature is -40 ° C and -5 ° C, the capacity decay of lithium-ion batteries at all SOC is very slow. When lithium-ion batteries are stored below 0 ° C, the above reaction is not significant, but it may still be the main mechanism leading to capacity degradation of lithium-ion batteries. This is because among the three aging mechanisms summarized in existing research, the aging of non active components is not considered under normal conditions, while Zhang et al. dissected and stored LiCo02/graphite batteries at low temperatures for 18 months and found no loss of anode or cathode active materials. In addition, Broussley et al. pointed out that a certain thickness of solid electrolyte interphase has a protective effect, which is consistent with the phenomenon observed in this study that the capacity of lithium-ion batteries is not easily decayed over time at low temperatures.
(3) When lithium-ion batteries with 0% S0C are stored at -40 ° C, -5 ° C, and 25 ° C, there is no capacity decay, and at all four test temperatures, the capacity of lithium-ion batteries is even higher than their original capacity after the initial 50 days of storage.
According to current research, this is the first time an increase in capacity has been observed in lithium cobalt oxide/graphite batteries during calendar aging tests. Of course, researchers have found similar observations in the aging experiments of nickel manganese diamond/graphite batteries and lithium iron phosphate/graphite. This increase in capacity is likely due to the temporary storage of lithium atoms in the anode suspension region, which can be eliminated through several complete cycles. However, when high SOC lithium-ion batteries are stored, the impact of SOC on the capacity of lithium-ion batteries is much greater than that of the anode hanging zone.
(4) At 50 ° C, lithium-ion batteries with a SOC of 0% only experience a 2% decrease in capacity after being stored for over 200 days. However, lithium-ion batteries with a SOC of 50% and 100% experience severe capacity degradation. After 125 days of storage, lithium-ion batteries with a SOC of 100% experience capacity degradation to less than 80% of their initial capacity.
This is because when lithium-ion batteries are stored at high temperatures, calendar aging is often the result of various aging mechanisms dominated by electrolyte decomposition and electrode active material reactions. The accelerated capacity degradation observed in lithium-ion batteries with a SOC of 100% after 60 days of storage is consistent with the experimental results of Zhang et al. This is because the capacity decay at this stage is mainly due to the severe loss of activated carbon in the anode, while before this stage, the capacity decay of lithium-ion batteries was mainly due to the generation of the intermediate phase in the solid electrolyte.
Based on the processing and analysis of test results, a mathematical model for the capacity degradation of lithium-ion batteries under cyclic aging and calendar aging can be further obtained. Due to the long duration of cyclic aging testing, the capacity degradation of lithium-ion batteries is the result of the combined effect of cyclic aging and calendar aging. Therefore, first establish a capacity decay model for calendar aging. The relative discharge capacity decay caused by calendar aging, QLoss, cal, is the result of the combined effect of temperature T, state of charge (SOC), and time t, namely:
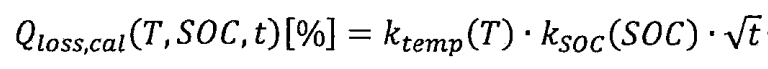
In the formula, Ktemp (T) represents the influence factor of temperature, Ksoc (SOC) represents the influence factor of state of charge, and the root sign T represents the influence factor of time. Among them, capacity decay has a square root relationship with time, because a large number of research results have shown that the generation of intermediate phases in solid electrolytes has a square root relationship with time. Due to the significant influence of the anode sag zone on the discharge capacity of lithium-ion batteries with a SOC of 0%, a reference temperature of 25 ° C and a reference SOC of 50% are set.
According to Arrhenius law, the effect of temperature on relative discharge capacity attenuation can be expressed as:
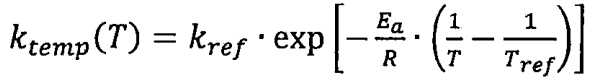
In the formula, Kref represents the influence factor at the reference temperature and SOC; Tref is the reference temperature, which is 298K; E α Is the activation energy, in J/mol; It is a universal gas constant with a value of 8.314J/(mol • K).
So the relative discharge capacity RDC can be expressed as:
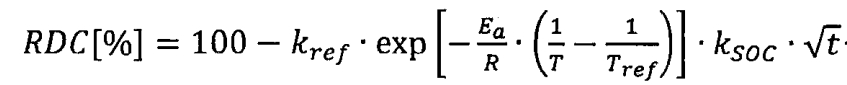
By substituting the reference temperature and SOC into the formula, we can obtain:
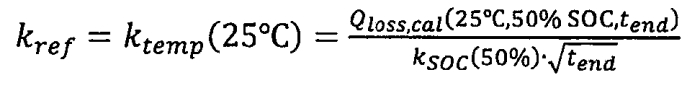
In the formula, tend is the end time of the test, measured in days. By fitting the test data, it can be concluded that when tend=240 days, Kref=0.186, E α= 32.434kJ/mol, Ksoc (SOC=100%)=1.249. Due to test data showing that calendar aging is independent of SOC at low temperatures, the activation energies at -40 ° C and -5 ° C were obtained to be 3.630 kJ/mol and 9.753 kJ/mol, respectively.
In addition, for lithium-ion batteries stored at 50 ° C with a SOC of 100%, it is necessary to add a power-law component to the model to better describe the accelerated decay phenomenon in the later stage of storage:
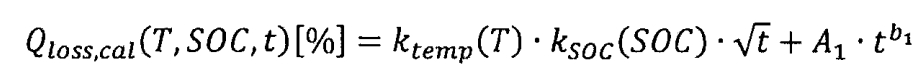
In the formula, A1 • t ^ b1 represents the capacity decay caused by severe loss of activated carbon in the anode. By fitting the test data, A1=2.271X10 ^ -4 and b1=2.286 were obtained.
