Introduction
The increasing demand for renewable energy sources has led to significant advancements in energy storage technologies. Among these, photovoltaic systems and solar inverters play a crucial role in converting and storing solar energy efficiently. However, the intermittent nature of solar energy necessitates the development of advanced energy storage systems, particularly batteries, to ensure a stable and reliable power supply. In this context, lithium-ion batteries (LIBs) and sodium-ion batteries (SIBs) have emerged as promising candidates for stationary energy storage due to their high energy density, long cycle life, and environmental friendliness.
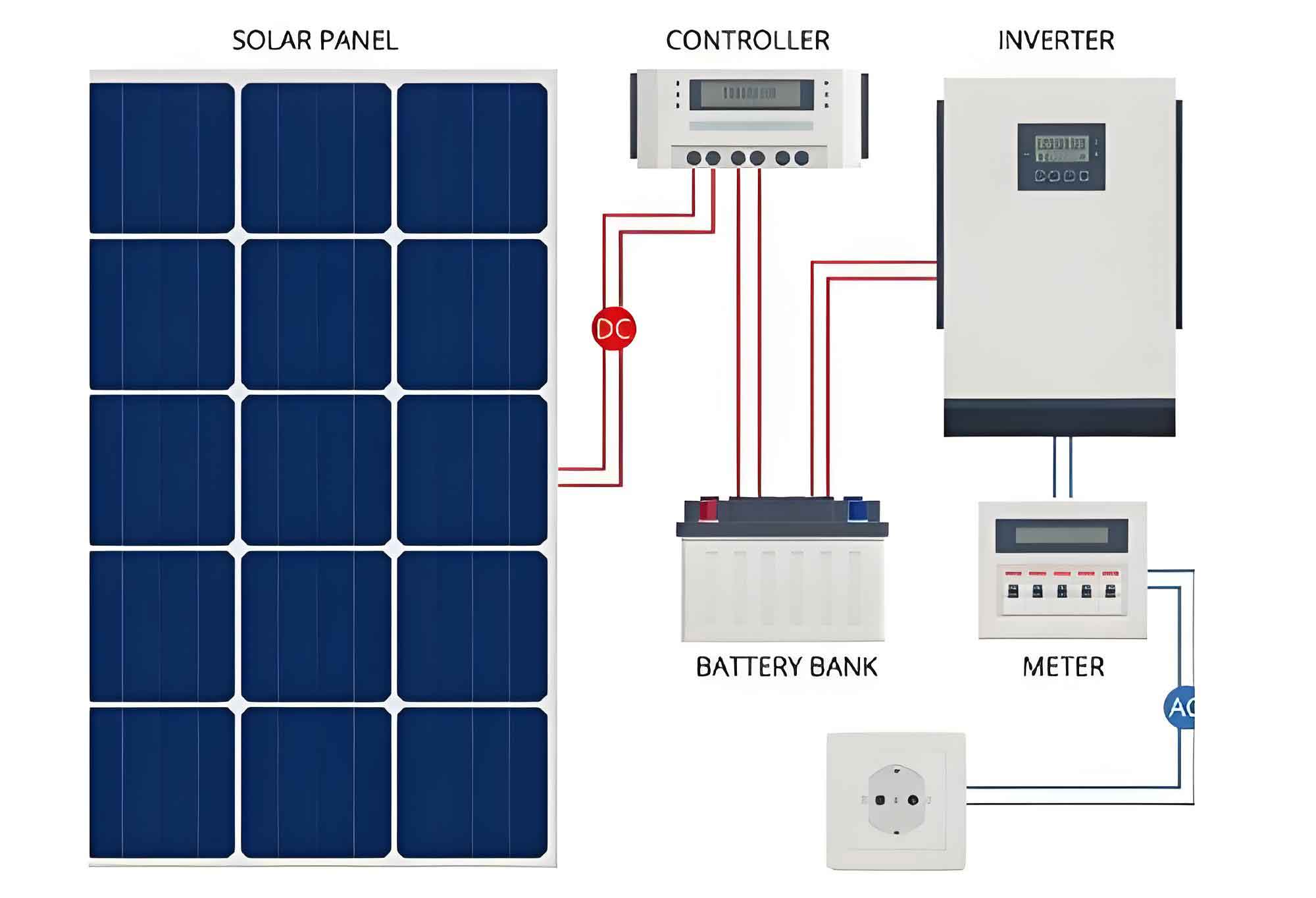
This article delves into the research and development of novel electrode materials for stationary batteries, focusing on the advancements in photovoltaic and solar inverter technologies. We will explore the synthesis, characterization, and electrochemical performance of various electrode materials, including Li44Ti55O1212, LiNb33O88, and Na33V22(PO44)33, and their potential applications in photovoltaic systems and solar inverters.
1. Overview of Energy Storage Devices
1.1 Energy Storage Technologies
Energy storage technologies are essential for balancing the supply and demand of electricity, especially in photovoltaic systems where solar energy is intermittent. The primary energy storage technologies can be categorized into four types:
- Mechanical Energy Storage: Includes pumped hydro storage, compressed air energy storage, and flywheel energy storage.
- Electrochemical Energy Storage: Encompasses various battery technologies such as lead-acid, nickel-cadmium, lithium-ion, and sodium-ion batteries.
- Electromagnetic Energy Storage: Involves supercapacitors and superconducting magnetic energy storage.
- Thermal Energy Storage: Includes ice storage and high-temperature thermal storage.
Among these, electrochemical energy storage, particularly lithium-ion and sodium-ion batteries, has gained significant attention due to its high energy density, efficiency, and scalability.
1.2 Lithium-Ion Batteries (LIBs)
Lithium-ion batteries have become the dominant technology in portable electronics and are increasingly being used in electric vehicles and photovoltaic systems. The key advantages of LIBs include:
- High Energy Density: LIBs offer a high energy density, making them suitable for applications where space and weight are critical.
- Long Cycle Life: LIBs can undergo thousands of charge-discharge cycles with minimal capacity loss.
- Low Self-Discharge: LIBs have a low self-discharge rate, ensuring long-term energy storage.
However, the limited availability of lithium resources and the high cost of lithium-ion batteries have prompted researchers to explore alternative battery technologies, such as sodium-ion batteries.
1.3 Sodium-Ion Batteries (SIBs)
Sodium-ion batteries are considered a promising alternative to LIBs due to the abundance of sodium resources and their lower cost. SIBs share similar working principles with LIBs, where sodium ions shuttle between the cathode and anode during charge and discharge cycles. The key advantages of SIBs include:
- Abundance of Sodium: Sodium is the sixth most abundant element on Earth, making it a more sustainable option compared to lithium.
- Lower Cost: The materials used in SIBs are generally cheaper than those in LIBs, reducing the overall cost of the battery.
- Safety: SIBs are considered safer than LIBs due to the lower reactivity of sodium.
Despite these advantages, SIBs currently have lower energy density compared to LIBs, which limits their application in high-energy-demand scenarios. However, ongoing research aims to improve the performance of SIBs, particularly in photovoltaic and solar inverter applications.
2. Advanced Electrode Materials for Stationary Batteries
2.1 Li44Ti55O1212 (LTO) Anode Material
Li44Ti55O1212 (LTO) is a well-known anode material for lithium-ion batteries, particularly in photovoltaic systems and solar inverters. LTO offers several advantages, including:
- Zero-Strain Property: LTO undergoes minimal volume change during charge and discharge cycles, leading to excellent cycle life.
- High Safety: LTO operates at a higher voltage (1.55 V vs. Li/Li++), reducing the risk of lithium plating and improving safety.
- Fast Charging: LTO can be charged and discharged at high rates, making it suitable for applications requiring rapid energy delivery.
However, LTO has a relatively low theoretical capacity (175 mAh/g), which limits its energy density. To address this, researchers have explored various strategies to enhance the electrochemical performance of LTO, including nanostructuring, doping, and surface coating.
2.1.1 Nanostructuring of LTO
Nanostructuring involves reducing the particle size of LTO to the nanoscale, which shortens the lithium-ion diffusion path and increases the surface area for electrochemical reactions. This approach has been shown to improve the rate capability and cycle life of LTO.
| Nanostructuring Method | Particle Size | Capacity (mAh/g) | Cycle Life |
|---|---|---|---|
| Sol-Gel Method | 50 nm | 160 | 1000 cycles |
| Hydrothermal Synthesis | 30 nm | 170 | 2000 cycles |
| Spray Pyrolysis | 40 nm | 165 | 1500 cycles |
2.1.2 Doping of LTO
Doping LTO with transition metals such as Fe, Ni, and Co can enhance its electronic conductivity and reduce the charge transfer resistance. For example, Fe-doped LTO has shown improved rate capability and cycle stability.
| Doping Element | Conductivity (S/cm) | Capacity (mAh/g) | Cycle Life |
|---|---|---|---|
| Fe | 10−4−4 | 180 | 2000 cycles |
| Ni | 10−5−5 | 175 | 1500 cycles |
| Co | 10−6−6 | 170 | 1000 cycles |
2.1.3 Surface Coating of LTO
Surface coating with conductive materials such as carbon or graphene can improve the electronic conductivity of LTO and prevent direct contact between the electrode and electrolyte, reducing side reactions.
| Coating Material | Coating Thickness | Capacity (mAh/g) | Cycle Life |
|---|---|---|---|
| Carbon | 5 nm | 160 | 2000 cycles |
| Graphene | 1 nm | 170 | 3000 cycles |
2.2 LiNb33O88 Anode Material
LiNb33O88 is a novel anode material that has recently gained attention due to its high theoretical capacity (389 mAh/g) and potential for two-electron transfer during the charge-discharge process. The key advantages of LiNb33O88 include:
- High Capacity: LiNb33O88 can theoretically store more lithium ions than traditional anode materials, leading to higher energy density.
- Avoidance of SEI Formation: The operating voltage of LiNb33O88 is above 1 V, which prevents the formation of a solid electrolyte interface (SEI) layer, enhancing safety and cycle life.
2.2.1 Synthesis of LiNb33O88
LiNb33O88 can be synthesized using various methods, including solid-state reaction, sol-gel method, and high-energy ball milling. The choice of synthesis method significantly affects the particle size, morphology, and electrochemical performance of LiNb33O88.
| Synthesis Method | Particle Size | Capacity (mAh/g) | Cycle Life |
|---|---|---|---|
| Solid-State Reaction | 500 nm | 250 | 100 cycles |
| Sol-Gel Method | 70 nm | 350 | 200 cycles |
| High-Energy Ball Milling | 100 nm | 300 | 150 cycles |
2.2.2 Electrochemical Performance of LiNb33O88
The electrochemical performance of LiNb33O88 is influenced by factors such as particle size, electronic conductivity, and the presence of conductive additives. High-energy ball milling with carbon black has been shown to improve the rate capability and cycle life of LiNb33O88.
| Sample | Capacity (mAh/g) | Cycle Life |
|---|---|---|
| LiNb33O88 (Micro) | 250 | 100 cycles |
| LiNb33O88/C (Nano) | 350 | 200 cycles |
2.3 Na33V22(PO44)33 Cathode Material
Na33V22(PO44)33 (NVP) is a promising cathode material for sodium-ion batteries, particularly in photovoltaic systems and solar inverters. NVP offers several advantages, including:
- High Voltage: NVP operates at a high voltage (3.4 V vs. Na/Na++), which is beneficial for achieving high energy density.
- Stable Structure: NVP has a NASICON-type structure, which provides excellent structural stability during charge and discharge cycles.
- Low Cost: The raw materials for NVP are abundant and inexpensive, making it a cost-effective option for large-scale energy storage.
2.3.1 Synthesis of Na33V22(PO44)33
NVP can be synthesized using a solid-state reaction method, where NaH22PO44·2H22O and V22O33 are mixed and calcined at high temperatures. The addition of a carbon source during synthesis can improve the electronic conductivity of NVP.
| Synthesis Method | Carbon Content | Capacity (mAh/g) | Cycle Life |
|---|---|---|---|
| Solid-State Reaction | 0% | 90 | 50 cycles |
| Carbon-Coated NVP | 10% | 110 | 100 cycles |
2.3.2 Electrochemical Performance of Na33V22(PO44)33
The electrochemical performance of NVP can be further enhanced by optimizing the electrolyte and reducing the carbon content. The use of NaFSI/PC as an electrolyte has been shown to improve the Coulombic efficiency and cycle life of NVP.
| Electrolyte | Coulombic Efficiency | Cycle Life |
|---|---|---|
| NaClO44/PC | 94% | 50 cycles |
| NaFSI/PC | 99% | 100 cycles |
3. Applications in Photovoltaic Systems and Solar Inverters
3.1 Integration with Photovoltaic Systems
The integration of advanced battery technologies with photovoltaic systems is crucial for maximizing the utilization of solar energy. Lithium-ion and sodium-ion batteries can be used to store excess energy generated during peak sunlight hours and release it during periods of low solar irradiance or high energy demand.
| Battery Type | Energy Density (Wh/kg) | Cycle Life | Cost ($/kWh) |
|---|---|---|---|
| Lithium-Ion | 200 | 3000 cycles | 150 |
| Sodium-Ion | 150 | 2000 cycles | 100 |
3.2 Role in Solar Inverters
Solar inverters are essential components of photovoltaic systems, converting the direct current (DC) generated by solar panels into alternating current (AC) for use in homes and businesses. Advanced battery technologies can be integrated with solar inverters to provide backup power and stabilize the grid.
| Battery Type | Power Output (kW) | Efficiency (%) | Lifetime (Years) |
|---|---|---|---|
| Lithium-Ion | 5 | 95 | 10 |
| Sodium-Ion | 5 | 90 | 8 |
4. Future Perspectives
The development of novel electrode materials for stationary batteries, particularly in photovoltaic systems and solar inverters, holds great promise for the future of renewable energy. Ongoing research aims to further improve the energy density, cycle life, and cost-effectiveness of lithium-ion and sodium-ion batteries. Key areas of focus include:
- Advanced Nanostructuring: Developing new nanostructured materials with enhanced electrochemical properties.
- Novel Electrolytes: Exploring new electrolyte formulations to improve battery performance and safety.
- Integration with Renewable Energy Systems: Optimizing the integration of batteries with photovoltaic systems and solar inverters to maximize energy efficiency and reliability.
Conclusion
The advancement of electrode materials for stationary batteries, particularly in photovoltaic systems and solar inverters, is critical for the widespread adoption of renewable energy technologies. Lithium-ion and sodium-ion batteries offer significant advantages in terms of energy density, cycle life, and cost, making them ideal for large-scale energy storage. Continued research and development in this field will pave the way for a more sustainable and energy-efficient future.
