Abstract
The increasing global demand for renewable energy sources has accelerated the development of energy storage technologies. Energy storage batteries, particularly lithium-ion batteries (LIBs) and sodium-ion batteries (SIBs), have emerged as promising solutions due to their high energy density, long cycle life, and environmental friendliness. However, the commercialization of these batteries faces several challenges, including high costs, limited resources, and safety concerns. To address these issues, the development of novel electrode materials with improved electrochemical performance is crucial. This review paper focuses on recent advancements in electrode materials for energy storage batteries, emphasizing their structures, electrochemical properties, and underlying mechanisms.

1. Introduction
Energy storage technologies play a vital role in the transition towards a sustainable energy future. Among various storage technologies, batteries, particularly LIBs and SIBs, have garnered significant attention due to their high energy density, long cycle life, and versatility. Despite their widespread adoption, LIBs face several limitations, including limited lithium resources, high costs, and potential safety hazards. In contrast, SIBs offer several advantages, such as abundant sodium resources, lower costs, and comparable electrochemical performance. Therefore, the development of novel electrode materials for both LIBs and SIBs is essential to enhance their performance, reduce costs, and improve safety.
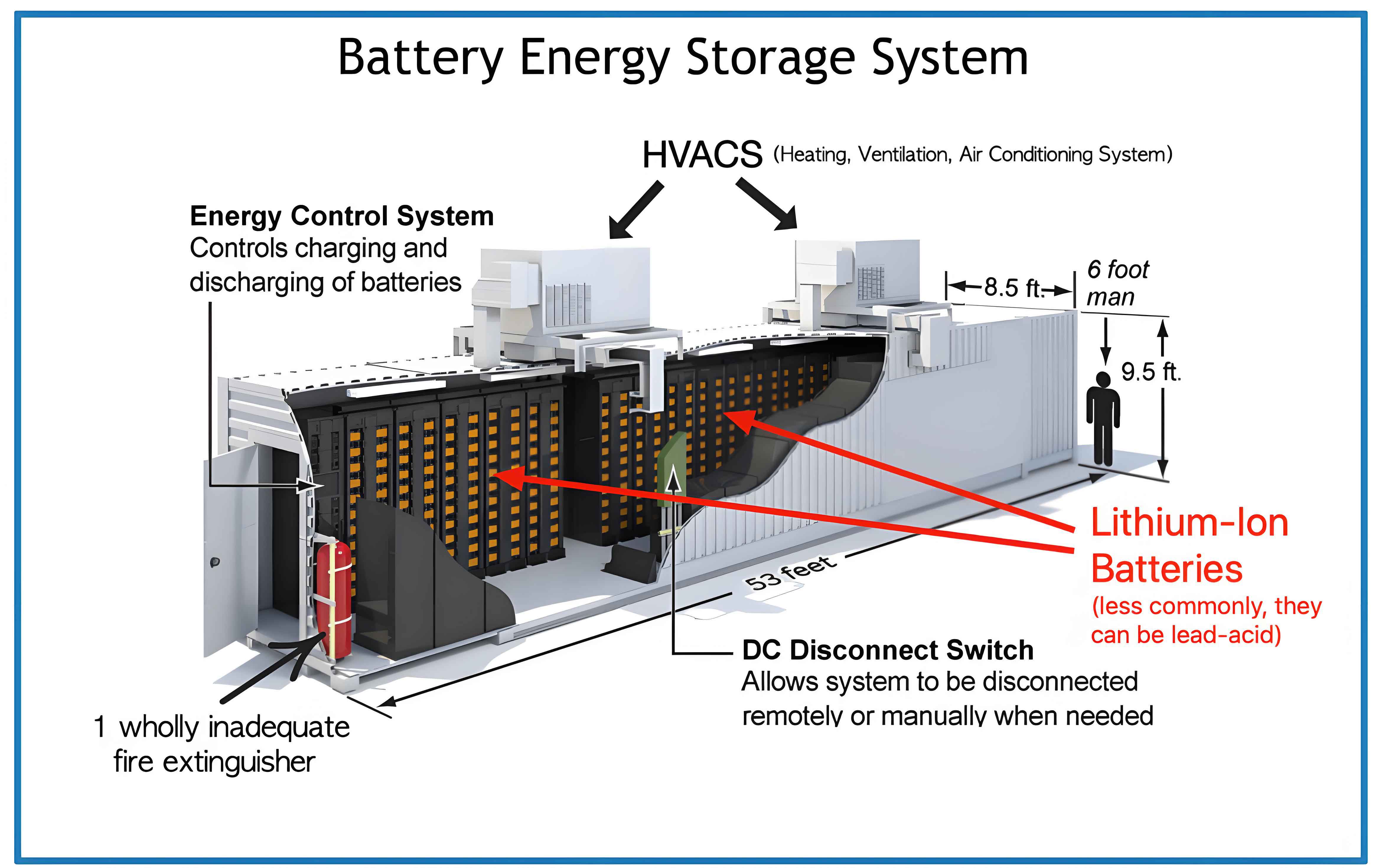
1.1 Overview of Energy Storage Batteries
Energy storage batteries can be classified into various types based on their chemistry and applications. The most prominent categories include LIBs, SIBs, lead-acid batteries, nickel-cadmium batteries, and flow batteries. Among these, LIBs and SIBs are considered the most promising due to their superior energy density and cycle life.
Table 1: Comparison of Different Energy Storage Battery Technologies
| Battery Type | Energy Density (Wh/kg) | Cycle Life (Cycles) | Cost ($/kWh) | Resource Availability |
|---|---|---|---|---|
| Lithium-Ion | 150-250 | 1000-5000 | 150-300 | Limited (Lithium) |
| Sodium-Ion | 100-150 | 2000-4000 | 50-150 | Abundant (Sodium) |
| Lead-Acid | 30-50 | 300-1000 | 30-80 | Abundant (Lead) |
| Nickel-Cadmium | 50-80 | 500-2000 | 100-200 | Moderate (Nickel, Cadmium) |
| Flow (Vanadium) | 25-50 | >10,000 | 200-400 | Abundant (Vanadium) |
1.2 Importance of Electrode Materials
The electrochemical performance of energy storage batteries is significantly influenced by the properties of their electrode materials. These materials must possess high specific capacity, good electronic and ionic conductivity, and structural stability during cycling. Additionally, the cost and environmental impact of electrode materials are also critical considerations.
2. Lithium-Ion Battery Electrode Materials
2.1 Cathode Materials
Cathode materials for LIBs typically consist of transition metal oxides or phosphates with layered, spinel, or olivine structures. The most commonly used cathode materials include lithium cobalt oxide (LCO), lithium nickel manganese cobalt oxide (NMC), and lithium iron phosphate (LFP).
Table 2: Common Cathode Materials for LIBs
| Material | Structure | Theoretical Capacity (mAh/g) | Voltage Range (V) | Advantages | Disadvantages |
|---|---|---|---|---|---|
| LiCoO2 (LCO) | Layered | 140 | 3.0-4.2 | High energy density, good cyclability | High cost, limited resources |
| LiNiMnCoO2 (NMC) | Layered | 160-200 | 3.0-4.3 | High energy density, good rate capability | Complex synthesis process |
| LiFePO4 (LFP) | Olivine | 170 | 2.5-3.7 | Good safety, low cost, abundant resources | Low energy density |
2.2 Anode Materials
Anode materials for LIBs can be divided into graphitic carbon, silicon-based materials, titanium-based materials, and conversion-type materials. Graphite is the most widely used anode material due to its low cost and good cyclability. However, silicon-based materials offer higher specific capacities but suffer from large volume changes during cycling.
Table 3: Common Anode Materials for LIBs
| Material | Theoretical Capacity (mAh/g) | Voltage Range (V) | Advantages | Disadvantages |
|---|---|---|---|---|
| Graphite | 372 | 0.01-0.25 | Low cost, good cyclability | Limited specific capacity |
| Silicon | 4200 | 0.005-0.4 | High specific capacity | Large volume changes, low cycle life |
| Li4Ti5O12 (LTO) | 175 | 1.0-2.5 | Zero-strain material, good safety | Limited specific capacity, high cost |
| SnO2 | 782 | 0.5-1.0 | High specific capacity | Large volume changes, low coulombic efficiency |
3. Sodium-Ion Battery Electrode Materials
3.1 Cathode Materials
SIB cathode materials must possess high sodium-ion diffusivity and stable structures during cycling. The most studied cathode materials include layered oxides, polyanionic compounds, and Prussian blue analogues.
Table 4: Common Cathode Materials for SIBs
| Material | Structure | Theoretical Capacity (mAh/g) | Voltage Range (V vs. Na/Na+) | Advantages | Disadvantages |
|---|---|---|---|---|---|
| NaFeO2 (Layered Oxide) | Layered | ~120 | 2.5-3.5 | High capacity, good rate capability | Air sensitivity |
| Na3V2(PO4)3 (NASICON) | NASICON | 117 | 3.4 and 1.6 | Good stability, long cycle life | Low energy density |
| Prussian Blue Analogues | Framework | 170 | 2.0-3.8 | High capacity, low cost | Water sensitivity |
3.2 Anode Materials
Anode materials for SIBs can be classified into carbon-based materials, alloy-based materials, and conversion-type materials. Hard carbon is the most commonly used anode material due to its high sodium storage capacity and good cycling stability.
Table 5: Common Anode Materials for SIBs
| Material | Theoretical Capacity (mAh/g) | Voltage Range (V vs. Na/Na+) | Advantages | Disadvantages |
|---|---|---|---|---|
| Hard Carbon | 200-350 | 0.01-0.5 | High capacity, good cycling stability | Low initial coulombic efficiency |
| Sn | 847 | 0.0-0.4 | High specific capacity | Large volume changes, low cycle life |
| Sb | 660 | 0.8-0.9 | Moderate volume changes | Lower capacity than Sn |
| Na3V2(PO4)3 (NASICON) | 117 (as anode) | >3.0 | High capacity, stable structure | Limited studies as anode material |
4. Novel Electrode Materials for Energy Storage Batteries
4.1 LiNb3O8 as Anode Material for LIBs
LiNb3O8 has recently emerged as a promising anode material for LIBs due to its high theoretical capacity and stable cycling performance. Its electrochemical behavior and structural evolution during cycling have been extensively studied.
4.1.1 Synthesis Methods
LiNb3O8 can be synthesized using various methods, including solid-state reaction, sol-gel, and hydrothermal synthesis. Solid-state reaction is the most commonly used method due to its simplicity and scalability.
Table 6: Synthesis Methods for LiNb3O8
| Method | Advantages | Disadvantages |
|---|---|---|
| Solid-State Reaction | Simple, scalable | High temperature required, long reaction time |
| Sol-Gel | Good homogeneity, controlled particle size | Complex process, solvents required |
| Hydrothermal | Good crystallinity, controlled morphology | High pressure required, long reaction time |
4.1.2 Electrochemical Performance
LiNb3O8 exhibits high specific capacities during cycling, with partial two-electron transfer reactions. However, its low electronic conductivity and large volume changes during cycling pose challenges.
4.2 Na3V2(PO4)3/C as Cathode Material for SIBs
Na3V2(PO4)3 (NVP) is a promising cathode material for SIBs due to its NASICON structure, high voltage platforms, and good structural stability. Carbon coating has been used to improve its electronic conductivity and cycling performance.
4.2.1 Synthesis and Characterization
NVP/C composites can be synthesized using a one-step solid-state reaction with sucrose as a carbon source. The structure and morphology of the synthesized materials are characterized using XRD, SEM, and TEM.
Table 7: Characterization Techniques for NVP/C Composites
| Technique | Purpose | Key Observations |
|---|---|---|
| XRD | Phase identification, crystal structure | Pure NASICON structure, no impurities |
| SEM | Morphology, particle size | Uniform particle size, porous structure |
| TEM | Microstructure, coating uniformity | Uniform carbon coating on NVP particles |
4.2.2 Electrochemical Performance
NVP/C composites exhibit high capacities, good rate capabilities, and long cycle lives as cathode materials for SIBs. The carbon coating significantly improves their electronic conductivity and cycling stability.
5. Conclusion and Future Perspectives
The development of novel electrode materials is crucial for enhancing the performance of energy storage batteries. Recent advancements in electrode materials for LIBs and SIBs have demonstrated improved electrochemical properties, including higher capacities, better rate capabilities, and longer cycle lives. However, further research is needed to address challenges related to cost, scalability, and environmental impact. Future directions include exploring novel materials, optimizing synthesis methods, and understanding underlying mechanisms to achieve more efficient and sustainable energy storage solutions.
