As a researcher deeply immersed in the field of energy storage, I have witnessed the transformative potential of solid-state batteries (SSBs) in revolutionizing next-generation energy systems. The quest for high-safety, high-energy-density batteries has driven extensive exploration into solid-state electrolytes (SSEs), where ion transport efficiency and electrode-electrolyte interface stability remain critical challenges. In this article, I synthesize recent advancements in leveraging nanowires to address these challenges, offering a detailed analysis of mechanisms, material innovations, and future directions.
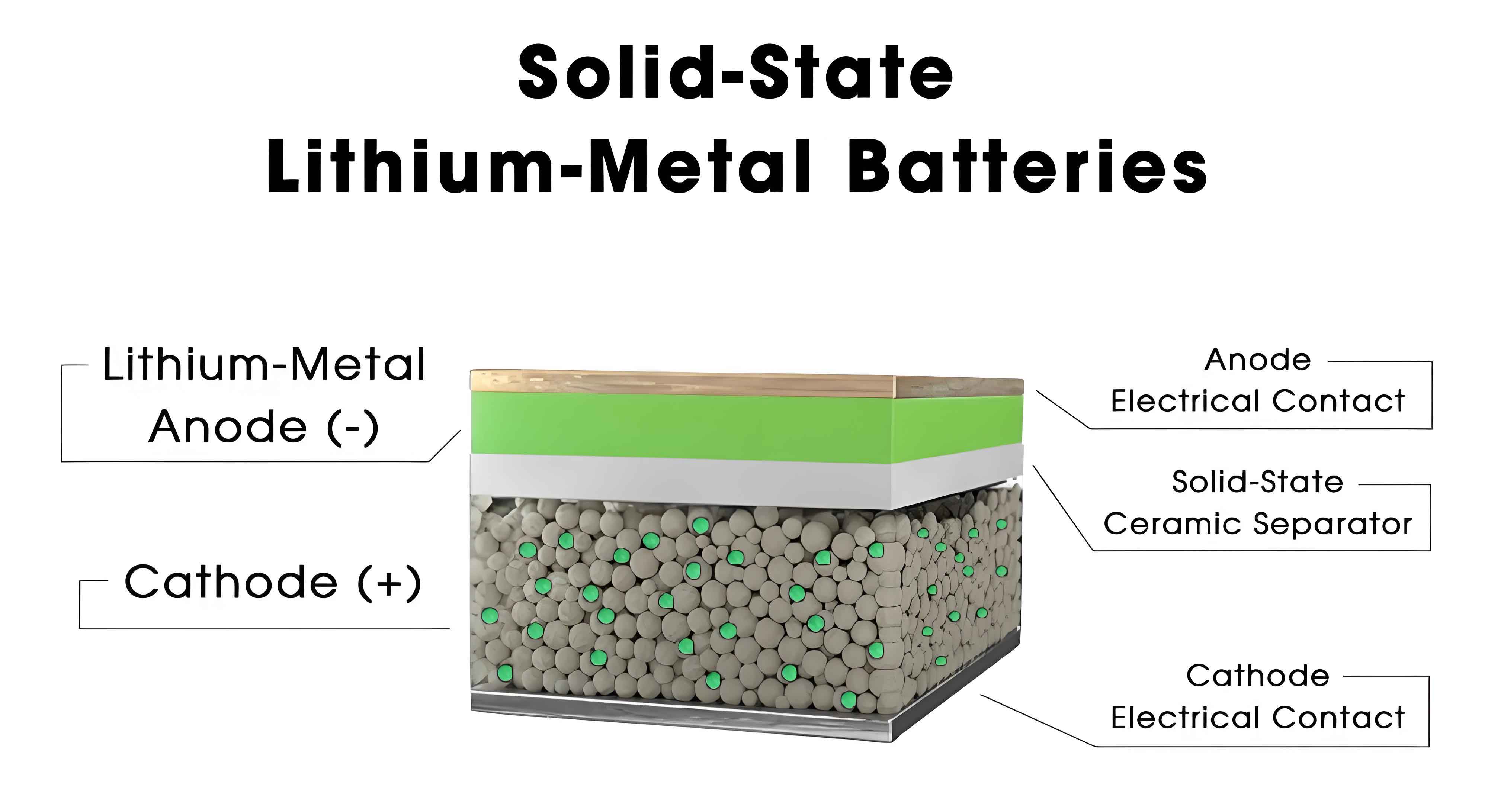
1. Introduction to Solid-State Batteries
Solid-state batteries replace flammable liquid electrolytes with solid alternatives, eliminating safety risks while enabling higher energy densities. SSEs are categorized into inorganic solid electrolytes (ISEs), polymer solid electrolytes (PSEs), and composite solid electrolytes (CSEs). Despite their promise, SSEs face bottlenecks:
- ISEs: High ionic conductivity but poor interfacial compatibility and mechanical brittleness.
- PSEs: Excellent flexibility but low room-temperature ionic conductivity (<10⁻⁶ S cm⁻¹).
- CSEs: Hybrid systems integrating polymers and inorganic fillers to balance performance.
Nanowires, with their high aspect ratio, tunable surface chemistry, and structural versatility, have emerged as game-changers for CSEs. Their unique properties—such as continuous ion transport pathways and interfacial modulation—position them as ideal candidates to overcome ion transport and interface limitations in SSBs.
2. Nanowire-Enhanced Ion Transport Mechanisms
The ionic conductivity (σ) of SSEs is governed by:σ=n⋅q⋅μσ=n⋅q⋅μ
where nn, qq, and μμ represent carrier concentration, charge, and mobility, respectively. Nanowires enhance nn and μμ through four key mechanisms:
2.1 Reducing Polymer Crystallinity
Polymers like polyethylene oxide (PEO) suffer from high crystallinity (>70%), which obstructs ion mobility. Introducing nanowires disrupts polymer chain alignment, increasing amorphous regions. For instance, CsPbI₃ nanowires reduce PEO crystallinity from 77.19% to 16.3%, elevating ionic conductivity by three orders of magnitude (Figure 1b). The relationship between crystallinity (XcXc) and conductivity follows:σ∝1Xcσ∝Xc1
Table 1: Impact of Nanowires on Polymer Crystallinity
| Polymer Matrix | Nanowire Type | Crystallinity Reduction | σ Enhancement (S cm⁻¹) |
|---|---|---|---|
| PEO | CsPbI₃ | 77.19% → 16.3% | 10⁻⁶ → 10⁻³ |
| PVDF | TiO₂ Nanotubes | 45% → 22% | 10⁻⁷ → 10⁻⁴ |
| PAN | LLTO | 60% → 30% | 10⁻⁸ → 10⁻⁴ |
2.2 Promoting Lithium Salt Dissociation
Nanowire surfaces functionalized with Lewis acid/base sites (e.g., -OH, -COOH) dissociate lithium salts (e.g., LiTFSI) via electrostatic interactions:LiTFSI→nanowireLi++TFSI−LiTFSInanowireLi++TFSI−
For example, YSZ nanowires with oxygen-rich defects increase free Li⁺ concentration, achieving σ = 0.267 mS cm⁻¹ at room temperature.
2.3 Mitigating Li⁺-Polymer Interactions
Hydrogen bonding between nanowires (e.g., boehmite nanowires) and polymer chains (e.g., PEO) reduces Li⁺-polymer coordination energy (EcoordEcoord):Ecoord=ELi-PEO−ELi-nanowireEcoord=ELi-PEO−ELi-nanowire
This weakens Li⁺ trapping, lowering activation energy (EaEa) for ion hopping.
2.4 Creating New Ion Transport Pathways
Active nanowires (e.g., LLTO, Li-HA-F) provide percolation networks for Li⁺ migration. Aligned LLTO nanowires achieve σ = 2.4×10⁻⁴ S cm⁻¹, tenfold higher than randomly dispersed counterparts. The percolation threshold (ϕcϕc) for conductivity follows:ϕc=13(dL)2ϕc=31(Ld)2
where dd and LL are nanowire diameter and length.
3. Nanowire-Driven Interface Engineering
The electrode-electrolyte interface in SSBs is prone to high impedance, dendrite growth, and parasitic reactions. Nanowires address these issues via:
3.1 Enhancing Interfacial Contact
- 3D Lithiophilic Scaffolds: Cu₃N nanowire arrays on garnet electrolytes reduce interfacial resistance to 4 Ω cm² by guiding uniform Li deposition.
- In Situ Polymerization: Acid-treated carbon nanotubes (ACNTP) induce DOL polymerization, forming seamless interfaces with cathodes.
3.2 Stabilizing Interfaces
- Anchoring Transition Metals (TMs): Asymmetric CSEs with CF₃-functionalized nanofibers suppress TM dissolution at high voltages (>4.5 V).
- SEI Engineering: Ca-PO₄-CO₃ nanowires catalyze TFSI⁻ decomposition, forming stable LiF-rich SEI layers.
Table 2: Nanowire Strategies for Interface Stabilization
| Challenge | Nanowire Solution | Outcome |
|---|---|---|
| Li Dendrite Growth | Li₃N@Cu Nanowires | CCD = 1.8 mA cm⁻² |
| Cathode-Electrolyte Gap | LLZO-PEO Composite | Interface Resistance < 50 Ω cm² |
| TM Dissolution | CF₃-Functionalized Fibers | Cycle Retention > 80% after 800 cycles |
4. Challenges and Future Perspectives
Despite progress, critical hurdles remain:
- Nanowire Dispersion: Agglomeration due to high surface energy limits interface effectiveness.
- Scalability: High-cost synthesis (e.g., CVD, electrospinning) hinders mass production.
- Mechanistic Understanding: Limited in situ evidence for Li⁺ transport pathways.
Future efforts should focus on:
- Advanced Characterization: Operando techniques like X-ray tomography and neutron depth profiling to visualize ion dynamics.
- Machine Learning: Accelerating nanowire design via predictive models for ionic conductivity and interface compatibility.
- Sustainable Synthesis: Low-energy methods (e.g., sol-gel, bio-inspired assembly) for eco-friendly scale-up.
5. Conclusion
Nanowires represent a paradigm shift in solid-state battery development, offering unparalleled control over ion transport and interfacial dynamics. By integrating nanowires into CSEs, researchers have achieved breakthroughs in ionic conductivity (>1 mS cm⁻¹), interfacial stability, and cycle life (>1000 cycles). However, translating lab-scale innovations to commercial SSBs demands interdisciplinary collaboration, scalable manufacturing, and deeper mechanistic insights. As we advance, the synergy between nanotechnology and solid-state electrochemistry will unlock unprecedented performance, paving the way for safer, higher-energy-density batteries.
