During the charging and discharging reaction, lithium-ion batteries release and absorb heat. At a constant temperature and pressure, lithium-ion batteries maintain thermodynamic equilibrium. Therefore, the heat released during the reaction process of lithium-ion batteries can be transformed using Gibbs free energy ΔG represents:
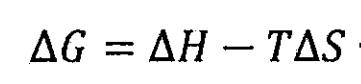
In the formula, AH is the enthalpy change of chemical reaction in lithium-ion batteries, measured in J; T is the absolute temperature of a chemical reaction, expressed in k; Δ S is the entropy change of a chemical reaction, measured in J/K. T Δ S is the chemical reaction heat Qr of lithium-ion batteries, that is:
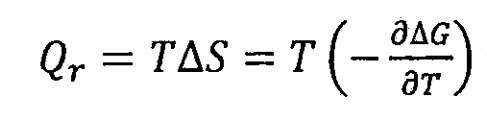
When lithium-ion batteries meet:
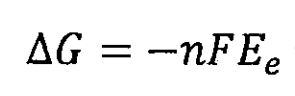
In the formula, n is the amount of electronic material exchanged during the chemical reaction process of the lithium-ion battery, in mol; F is the Faraday constant, measured in C/mol; Ee is the electromotive force of a lithium-ion battery, measured in J/C.
In the formula, it can be transformed into:
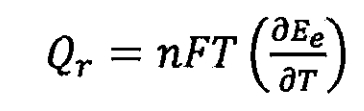
However, the electrochemical polarization of lithium-ion batteries during the charging and discharging process can cause the actual terminal voltage of the lithium-ion battery to deviate from the equilibrium electromotive force (electrode potential), accompanied by the generation of heat. This heat is recorded as the polarization heat Qp of lithium-ion batteries, which is due to energy loss caused by electrochemical polarization. In addition, the chemical reactions of lithium-ion batteries are accompanied by side reactions, self discharge, and other phenomena caused by the decomposition of electrode materials and electrolytes. There is also heat generated, which is recorded as side reaction heat<2S. Due to the presence of resistance inside lithium-ion batteries, Joule heat, also known as Ohmic internal resistance heat Qj, is generated.
Therefore, the total heat generation Qt of lithium-ion batteries during the charging and discharging process is:
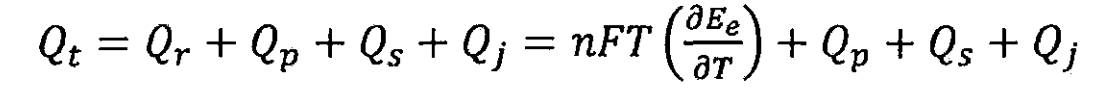
When overcharging lithium-ion batteries, side reactions may occur, but once these reactions occur, gas will be produced, endangering the safety of lithium-ion batteries. Therefore, it is believed that under normal circumstances, the heat of side reactions during lithium-ion battery charging is zero. When the battery is discharged, the heat generated due to the decomposition of electrode materials and electrolytes is very small, which is negligible compared to other heat sources; When discharging, the chemical reaction heat of lithium-ion batteries is positive, and the internal resistance of lithium-ion batteries also generates Joule heat. When the self discharge capacity of lithium-ion batteries is large, these heat cannot be ignored, but for lithium-ion batteries, the self discharge capacity is low enough to ignore the side reaction heat during discharge. Therefore, for lithium-ion batteries, the heat of side reactions can be ignored, and the total heat generated during the charging and discharging process of lithium-ion batteries can be simplified as:
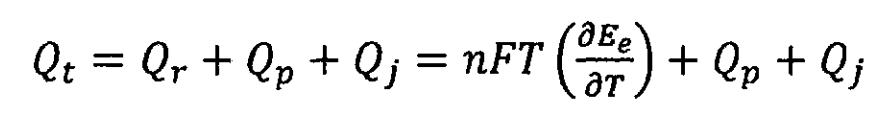
Namely, chemical reaction heat Qr, polarization heat Qp, and Ohmic internal resistance heat Qj.
(1) Heat of chemical reaction
During the charging and discharging process of lithium-ion batteries, lithium ions are embedded and removed between the positive and negative electrode materials. The heat generated during this process is called chemical reaction heat, which is reversible. It absorbs heat during charging and generates heat during discharge. When charging, the heat of chemical reaction per unit time can be expressed as:
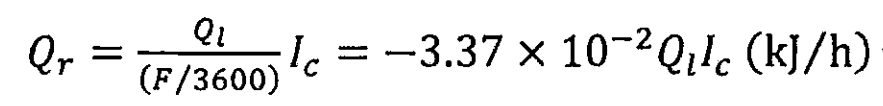
In the formula, Qt is the total heat generated by the chemical reaction in kJ/mol; Ic is the charging current, in amperes.
(2) Polarization heat
The reason why polarization causes the actual terminal voltage of lithium-ion batteries to deviate from the theoretical electromotive force is that the diffusion and movement of atoms during the reaction process of lithium-ion batteries require energy. The chemical diffusion coefficient of lithium ions is relatively low, at 5×10 ^ -9cm ^ 2/s. Therefore, the diffusion and movement of lithium ions in the electrode are the main reasons for polarization heat generation. In addition, as the number of cycles increases, the degradation of electrode surface active substances will also increase polarization. When charging, the polarization heat per unit time can be expressed as:
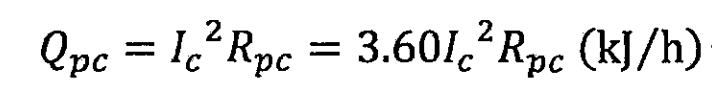
In the formula, Rpc is the polarization resistance of the lithium-ion battery during charging, in Ω, and Id can be obtained from the formula A.
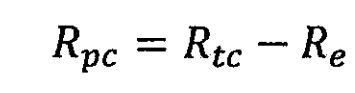
In the formula, Rtc is the total resistance of the lithium-ion battery during charging, and Re is the internal resistance of the lithium-ion battery itself, measured in Ω. Polarization heat is manifested as Joule heat and cannot be directly calculated. It can only be calculated by separating the polarization resistance from the total resistance of lithium-ion batteries.
(3) Ohmic internal resistance heat
Ohmic internal resistance heat is the heat generated by the current passing through the Ohmic resistance composed of various materials inside the lithium-ion battery, manifested as Joule heat, which is positive during the charging and discharging process of the lithium-ion battery. The expression for Ohmic internal resistance heat per unit time is as follows:
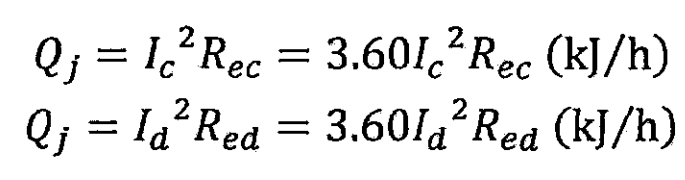
In the formula, Rec and Red are the ohmic resistance of the lithium-ion battery during charging and discharging, in Ω, and Id is the discharge current, in A.
Overall, the thermal generation inside lithium-ion batteries is a complex process that is related to the rate of electrochemical reactions over time and temperature, as well as the movement of current in lithium-ion batteries. Lithium ion batteries generate heat through reversible entropy, resistance dissipation, relaxation of battery concentration gradients, and chemical reactions. For this article, the main purpose is to study the thermal simulation and management of lithium-ion battery packs. Therefore, it can be considered that a single lithium-ion battery is homogeneous, and heat is uniformly generated on a single lithium-ion battery. The heat generation rate model proposed by Bemardi et al. can also be used to estimate the heat generation rate of the battery:
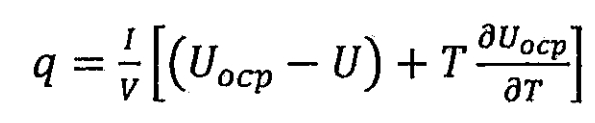
In the formula, I is the charge and discharge current of the lithium-ion battery, with discharge being positive and charge being negative, in units of A; V is the volume of lithium-ion batteries, in m ^ 3; Uocp is the open circuit voltage of the battery, i.e. the electromotive force Ee of the lithium-ion battery, measured in volts; U is the terminal voltage of the lithium-ion battery, in volts. According to Ohm’s law, Uocp-U can be replaced by the product of current and the total internal resistance of a lithium-ion battery. Therefore, the formula can be transformed into:
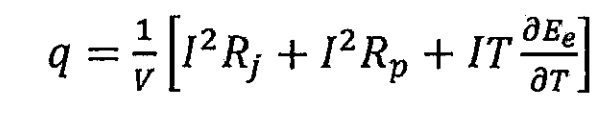
In the formula, I ^ 2Rj is the Ohmic internal resistance heat term, I ^ 2Rp is the polarization heat term, and IT (Ee)/T is the chemical reaction heat term.
From this, it can be seen that during the normal operation of lithium-ion batteries, the heat generation rate of lithium-ion batteries during the charging and discharging process is mainly affected by the charging and discharging current, the ohmic resistance, polarization resistance, temperature of lithium-ion batteries, and the coefficient of change of electromotive force temperature.
