In the context of global efforts to advance energy transition and address climate change, the development and utilization of renewable energy sources such as solar and wind power have made significant progress. Lithium-ion battery energy storage systems, with their outstanding advantages of high energy density, fast response speed, and long cycle life, have become a key technology for solving renewable energy integration issues and enhancing grid stability. They are widely applied in generation-side, grid-side, and user-side scenarios. Despite these benefits, the frequent occurrence of safety incidents in recent years, particularly fire and explosion accidents, has resulted in severe casualties and substantial property losses, drawing heightened attention from all sectors of society to the safety of these systems.
As a researcher focused on energy storage safety, I have extensively studied the underlying causes and mitigation strategies for these hazards. In this article, I will analyze the risk factors, explore the evolution mechanisms, and discuss prevention and control technologies, aiming to enhance the safety and reliability of lithium-ion battery energy storage systems.
Analysis of Fire and Explosion Risk Factors in Lithium-ion Battery Energy Storage Systems
Thermal Runaway of Batteries
Thermal runaway is the primary root cause of fire and explosion incidents in lithium-ion battery energy storage systems. When heat generated internally cannot be dissipated promptly, it leads to a rapid temperature rise, triggering a series of irreversible chemical reactions that ultimately result in thermal runaway. The inducing factors are complex and diverse, encompassing both internal and external elements.
From internal perspectives, impurities introduced during cell manufacturing, microstructural defects in electrode materials, and instability of electrolytes can all initiate internal short circuits during battery operation, leading to thermal runaway. When an internal short circuit occurs, the current increases sharply, generating substantial Joule heat, which rapidly elevates the battery temperature, as shown in Equation (1).
$$ Q = I^2 R T $$
where: \( Q \) is heat (J), \( I \) is current (A), \( R \) is resistance (Ω), and \( T \) is time (s).
As temperature rises, the chemical reactivity between electrode materials and electrolytes intensifies, releasing more heat and forming a vicious cycle that culminates in thermal runaway. Key chemical reactions include the following two types.
(1) Reaction between negative electrode and electrolyte:
$$ \text{LiC}_6 + x\text{PF}_5 \rightarrow \text{LiF} + \text{LiPF}_y + \text{C}_6 + (x-y)\text{PF}_3 + (x-1)\text{F}_2 $$
(2) Reaction between positive electrode material and electrolyte (using LiCoO\(_2\) as an example):
$$ \text{LiCoO}_2 + \text{C}_2\text{H}_4\text{O}_3 \rightarrow \text{Li}_2\text{CO}_3 + \text{Co}_3\text{O}_4 + \text{CO}_2 + \text{C} $$
External factors such as overcharge, over-discharge, overheating, and mechanical abuse easily induce thermal runaway in lithium-ion batteries. Overcharge can cause lithium dendrites to pierce the separator, leading to short circuits; over-discharge results in irreversible structural changes in electrode materials, increasing the risk of runaway; in high-temperature environments, heat generation surges, and insufficient dissipation can trigger runaway; mechanical abuse like collisions or compression damages internal battery structures, potentially causing electrode short circuits and ultimately thermal runaway.
Electrical Faults
Electrical faults are a significant risk source for fire and explosion in lithium-ion battery energy storage systems. The causes of short-circuit faults are complex. Deviations in voltage and current monitoring and control by the Battery Management System (BMS) can lead to overcharge or over-discharge of batteries, inducing internal short circuits. Electrical lines, after long-term operation, may age or degrade in insulation performance, with insulation layers damaged under temperature and humidity influences, also causing line short circuits. Loose or poorly connected components can overheat locally under high currents, similarly potentially leading to short circuits. Table 1 summarizes the proportion statistics for short-circuit fault causes.
| Fault Type | Proportion /% | Typical Case |
|---|---|---|
| BMS Monitoring Deviation | 35 | Unit battery overcharge leading to internal short circuit |
| Line Insulation and Aging | 40 | Line breakdown in environments with relative humidity ≥ 60% |
| Loose Connection Components | 20 | High current causing contact point overheating and melting |
| Others (e.g., Lightning Strike) | 5 | Surge voltage breaking down battery insulation layer |
Design and Installation Defects
Unreasonable system design embeds safety hazards in lithium-ion battery energy storage systems. Mismatched battery selection for actual application scenarios, such as using low-rate discharge batteries in high-power demand situations, can cause batteries to operate under high load for extended periods, accelerating aging and increasing thermal runaway risk. Improper design of battery series-parallel configurations may lead to voltage and current imbalances between battery groups, causing some batteries to overcharge or over-discharge, affecting battery lifespan and safety.
Inadequate thermal management system design is another critical safety hazard. If the thermal management system cannot effectively control battery operating temperature, it may fail to dissipate heat promptly during high heat generation, leading to excessive temperature and thermal runaway. Conversely, in low-temperature environments, ineffective heating can reduce battery performance and elevate safety risks.
Evolution Mechanisms of Fire and Explosion in Lithium-ion Battery Energy Storage Systems
Chain Reactions Triggered by Thermal Runaway
When thermal runaway occurs in a lithium-ion battery, it triggers a series of complex chain reactions. First, the internal temperature rises, causing decomposition of the Solid Electrolyte Interface (SEI) film on the negative electrode surface. When temperature exceeds a certain threshold, the SEI film begins to decompose, releasing heat and flammable gases such as carbon monoxide and hydrogen. These flammable gases mix with air to form combustible mixtures, which can ignite upon encountering an ignition source.
After SEI film decomposition, negative electrode materials directly contact the electrolyte, undergoing vigorous redox reactions that release substantial additional heat. As reactions progress, battery temperature continues to rise, and internal pressure increases. When pressure exceeds the casing’s承受极限, the battery casing ruptures, ejecting internal combustible materials and high-temperature gases, forming jet fires that induce thermal runaway in adjacent batteries. This creates a连锁反应, leading to rapid fire spread.
Fire Propagation and Spread Mechanisms
In lithium-ion battery energy storage systems, fire propagation and spread primarily occur through three modes: thermal radiation, thermal conduction, and thermal convection, in addition to flame propagation.
In flame propagation, the laminar flame speed formula is:
$$ S_L = \frac{\dot{m}”}{\rho_u} $$
where: \( S_L \) is laminar flame speed (cm/s), \( \dot{m}” \) is mass burning rate (kg/(m²·s)), and \( \rho_u \) is density of unburned mixture (kg/m³).
The turbulent flame speed is:
$$ S_T = S_L (1 + \alpha \text{Re}^n) $$
where: \( S_T \) is turbulent flame speed (cm/s), \( S_L \) is laminar flame speed (cm/s), \( \alpha \) is turbulence influence coefficient, \( \text{Re} \) is Reynolds number, and \( n \) is Reynolds number exponent, taken as 0.25 in this context.
Thermal radiation follows the Stefan-Boltzmann law:
$$ Q_{\text{rad}} = \varepsilon \sigma A (T_1^4 – T_2^4) $$
where: \( Q_{\text{rad}} \) is heat transfer by radiation (W), \( \varepsilon \) is surface emissivity (reflecting object’s radiation capability), \( \sigma \) is Stefan-Boltzmann constant (W/(m²·K⁴)), with a fixed value of \( 5.67 \times 10^{-8} \, \text{W/(m²·K⁴)} \), \( A \) is radiation surface area (m²), \( T_1 \) is absolute temperature of high-temperature object (K), and \( T_2 \) is absolute temperature of low-temperature object (K).
Long-distance radiation can be estimated using:
$$ q = \frac{Q_{\text{total}} \varepsilon}{4 \pi r^2} $$
where: \( q \) is heat flux density (W/m²), \( Q_{\text{total}} \) is total radiative heat flow (W), and \( r \) is distance from radiation source to irradiated surface (m).
Thermal conduction is based on Fourier’s law:
$$ Q_{\text{cond}} = -k A_{\text{cond}} \frac{dT}{dx} $$
where: \( Q_{\text{cond}} \) is heat transfer by conduction (W), \( k \) is thermal conductivity of material (W/(m·K)), \( A_{\text{cond}} \) is cross-sectional area for conduction (m²), and \( \frac{dT}{dx} \) is temperature gradient (K/m).
Thermal convection heat transfer is calculated via Newton’s law of cooling:
$$ Q_{\text{conv}} = h A_{\text{conv}} (T_w – T_f) $$
where: \( Q_{\text{conv}} \) is heat transfer by convection (W), \( h \) is convective heat transfer coefficient (W/(m²·K)), \( A_{\text{conv}} \) is convection surface area (m²), \( T_w \) is absolute temperature of solid wall (K), and \( T_f \) is absolute temperature of fluid (K).
For natural convection, the heat transfer coefficient is often calculated with:
$$ \text{Nu} = C (\text{Gr} \cdot \text{Pr})^n $$
where: \( \text{Nu} \) is Nusselt number, \( C \) is empirical constant, \( \text{Gr} \) is Grashof number, \( \text{Pr} \) is Prandtl number, and \( n \) is empirical exponent.
For example, given laminar flame speed \( S_L = 10 \, \text{cm/s} \), \( \alpha = 0.5 \), \( \text{Re} = 100 \), \( n = 0.25 \), turbulent flame speed \( S_T \) is computed:
$$ S_T = S_L (1 + \alpha \text{Re}^n) = 10 \times (1 + 0.5 \times 100^{0.25}) = 10 \times (1 + 0.5 \times 3.162) \approx 25.81 \, \text{cm/s} $$
Thus, turbulent flame speed is significantly higher than laminar speed.
When a battery fire occurs, flames and high-temperature batteries heat surrounding objects via radiation; conduction through metal connectors induces thermal runaway in adjacent batteries; convection via hot smoke and ventilation ducts transfers heat and disperses flammable gases—all accelerating fire spread.
Explosion Formation Conditions and Process
Explosions in lithium-ion battery energy storage systems require specific conditions: flammable gases must mix with air in appropriate proportions and encounter an ignition source with sufficient energy. During thermal runaway, large quantities of flammable gases such as hydrogen, carbon monoxide, and alkanes are released. These gases accumulate in the battery compartment, mixing with air to form combustible mixtures. When the mixture concentration reaches within the explosion limits, ignition sources like electrical sparks or open flames can trigger an explosion.
To assess whether a flammable gas mixture is within the explosion risk range, Le Chatelier’s rule can be applied. For multiple flammable gases mixed, the lower explosion limit \( \text{LEL}_{\text{mix}} \) and upper explosion limit \( \text{UEL}_{\text{mix}} \) are given by:
$$ \text{LEL}_{\text{mix}} = \frac{100}{\frac{y_1}{\text{LEL}_1} + \frac{y_2}{\text{LEL}_2} + \ldots + \frac{y_n}{\text{LEL}_n}} $$
where: \( \text{LEL}_{\text{mix}} \) is lower explosion limit of mixed flammable gases (% by volume), \( y_1, y_2, \ldots, y_n \) are volume fractions of individual gases (%), and \( \text{LEL}_1, \text{LEL}_2, \ldots, \text{LEL}_n \) are lower explosion limits of individual gases (% by volume).
$$ \text{UEL}_{\text{mix}} = \frac{100}{\frac{y_1}{\text{UEL}_1} + \frac{y_2}{\text{UEL}_2} + \ldots + \frac{y_n}{\text{UEL}_n}} $$
where: \( \text{UEL}_{\text{mix}} \) is upper explosion limit of mixed flammable gases (% by volume), and \( \text{UEL}_1, \text{UEL}_2, \ldots, \text{UEL}_n \) are upper explosion limits of individual gases (% by volume).
Different flammable gases have varying Minimum Ignition Energy (MIE) requirements, with typical data shown in Table 2.
| Flammable Gas Type | Minimum Ignition Energy /mJ |
|---|---|
| Hydrogen | 0.02 |
| Methane | 0.28 |
| Carbon Monoxide | 0.021 |
Fire and Explosion Prevention and Control Technologies for Lithium-ion Battery Energy Storage Systems
Battery Material and Design Improvements
To enhance the safety of lithium-ion batteries from the source, developing high-safety battery materials is crucial.
For positive electrode materials, lithium iron phosphate (LFP) is widely used in energy storage due to its excellent thermal stability and safety. Compared to traditional materials like lithium cobalt oxide, LFP maintains structural stability at high temperatures, significantly reducing thermal runaway risk in lithium-ion battery systems.
For negative electrode materials, silicon-based materials are considered a future direction due to their high theoretical specific capacity. However, volume changes during charge-discharge cycles can damage electrode structures, affecting battery performance and safety. Researchers are improving stability and cycle performance through nanostructure design and material composites.
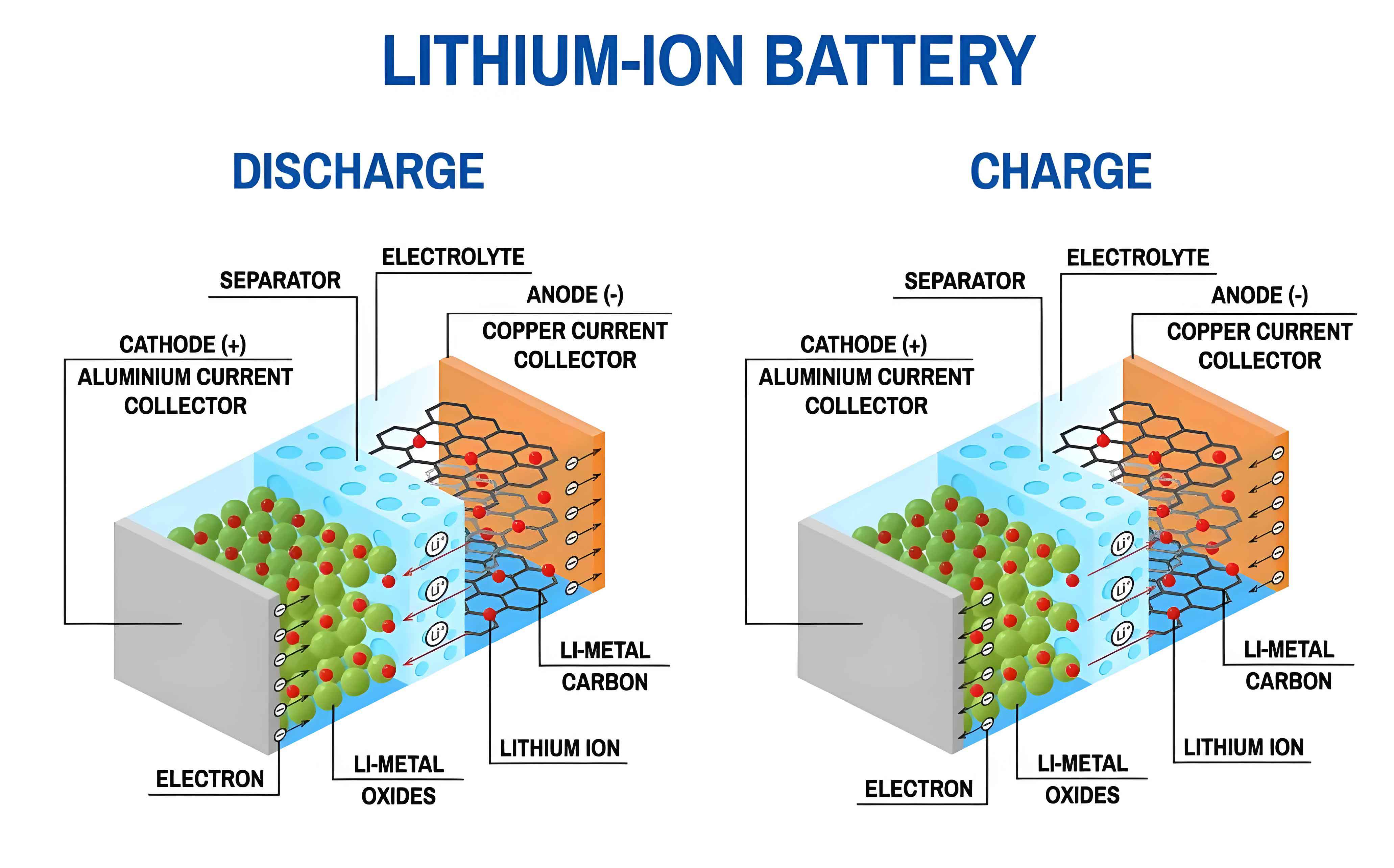
Monitoring and Early Warning Technologies
Real-time monitoring and early warning are key to preventing and controlling risks in lithium-ion battery energy storage systems. Voltage and current monitoring assess battery charge-discharge states and health, providing early warnings for overcharge, over-discharge, and other issues. Temperature sensors monitor battery module and compartment temperatures in real-time, triggering cooling and alarms when thresholds are exceeded. Pressure sensors detect abnormal internal battery pressure, activating ventilation and pressure relief measures. Gas composition monitoring uses sensors like catalytic combustion or semiconductor types to detect concentrations of hydrogen and carbon monoxide, issuing warnings when flammable gases approach explosion下限 thresholds. Table 3 outlines monitoring parameter threshold ranges.
| Monitoring Parameter | Normal Operation Range | Abnormal Warning Range |
|---|---|---|
| Voltage /V (single cell) | 2.5~4.2 | <2.5 (over-discharge) or >4.2 (overcharge) |
| Current | 0~120% of rated current | >120% of rated current (overcurrent) |
| Temperature (battery module) /℃ | 25~45 | >45 (temperature rise warning) |
| Temperature (compartment) /℃ | 20~35 | >35 (environment overheating) |
| Pressure (battery internal) /MPa | 0~0.1 | >0.1 (pressure anomaly) |
| Hydrogen Volume Fraction /% | 0~0.04 | >0.04 |
| Carbon Monoxide Volume Fraction /% | 0~0.005 | >0.005 |
Optimized Thermal Management Systems
Thermal management systems should be optimized based on the operational characteristics of lithium-ion batteries. Combined liquid-air cooling technologies can be employed, using Proportional-Integral-Derivative (PID) algorithms to adjust coolant flow and fan speed, maintaining temperature differences within battery stacks within ±2 ℃. Phase change materials embedded between battery components absorb sudden heat via latent heat of fusion, coupled with metal heat sinks to enhance thermal conductivity. Temperature monitoring points are distributed in a grid pattern, with real-time data fed into the BMS for predicting temperature trends. The ideal operating temperature range for lithium-ion batteries is 25~45 ℃. When temperature exceeds 40 ℃, the startup power of the cooling system correlates linearly with temperature rise, as shown in Equation (13).
$$ P = K \times (T – T_0) $$
where: \( P \) is startup power of cooling system (W), \( K \) is thermal radiation coefficient, taken as 15 W/℃, \( T \) is real-time battery temperature (℃), and \( T_0 \) is reference temperature, taken as 40 ℃.
The curve in Figure 3 is fitted from experimental data; when temperature surpasses critical values, cooling power can be increased to prevent heat accumulation.
Fire Suppression and Explosion Prevention Measures
When fire or explosion occurs, efficient suppression and prevention measures can minimize losses. Among fire suppressants, water mist offers good cooling with minimal water damage, while perfluorohexanone and heptafluoropropane provide high灭火效率 but at higher costs, all commonly used for lithium-ion battery fires. For explosion prevention, battery compartments should adopt explosion-proof structures. The vent area can be precisely calculated using Equation (14).
$$ A = 10 C’ V^{2/3} $$
where: \( A \) is vent area of battery compartment (m²), \( C’ \) is volume of battery compartment (m³), taken as 80 m³ in this example, and \( V \) is venting coefficient or related correction factor, determined based on battery type, flammable gas properties, and safety standards.
For instance, given battery compartment volume \( C’ = 80 \, \text{m}^3 \) and assuming \( V = 1 \) (here \( V \) parameter is unspecified, temporarily set to 1), substituting into Equation (14):
$$ A = 10 \times 80 \times 1^{2/3} = 10 \times 80 \times 1 = 800 \, \text{m}^2 $$
Thus, vent area is 800 m²; in practice, adjustments based on specific \( V \) values and standards are necessary to ensure effective pressure relief.
Conclusion
Lithium-ion battery energy storage systems, as core支撑 technologies for energy transition and new power system construction, have safety performance directly impacting energy security and public safety. They are a critical cornerstone for ensuring stable operation of energy systems. My research identifies battery thermal runaway, electrical faults, design and installation defects, and environmental abnormalities as the four core诱因 for fire and explosion incidents. I have systematically elucidated the internal mechanisms of thermal runaway chain reactions triggering, cross-unit fire spread, and explosion shockwave formation. Addressing these risks, the industry has developed a multi-dimensional prevention and control technology体系, covering key areas such as battery material innovation, structural design optimization, thermal management system upgrades, multi-parameter monitoring and early warning, and efficient fire suppression and explosion prevention device研发. However, current prevention technologies still have shortcomings. Future research should target these gaps to drive continuous iteration and升级 of prevention technologies, building a robust屏障 for the large-scale and safe application of lithium-ion battery energy storage systems. Throughout this analysis, the importance of proactive measures and ongoing innovation cannot be overstated for the sustainable deployment of lithium-ion battery technologies in global energy infrastructure.
To further elaborate on risk mitigation, I emphasize that integrating advanced sensing technologies with artificial intelligence can enhance predictive maintenance for lithium-ion battery systems. For example, machine learning algorithms can analyze historical voltage, current, and temperature data to forecast potential failures before they escalate. Additionally, standardization of safety protocols across regions is vital to ensure consistent protection levels. Another aspect is the recycling and second-life applications of lithium-ion batteries, which, while not directly covered here, influence overall system safety by managing end-of-life risks. In my view, collaborative efforts among researchers, manufacturers, and policymakers are essential to address these multifaceted challenges. By fostering innovation in materials science, such as solid-state electrolytes that reduce flammability, we can fundamentally transform the safety landscape of lithium-ion battery energy storage. Moreover, regular safety drills and training for personnel operating these systems can prevent human errors that often contribute to incidents. As we advance, continuous monitoring of emerging technologies like sodium-ion or flow batteries may offer complementary solutions, but lithium-ion batteries will likely remain predominant in the near term, necessitating relentless focus on their safe deployment. Ultimately, the goal is to balance performance and safety, enabling lithium-ion battery energy storage to fulfill its potential in supporting renewable energy integration and grid resilience worldwide.
In summary, the journey toward safer lithium-ion battery energy storage systems involves a holistic approach encompassing design, operation, and regulatory frameworks. My analysis underscores that while significant progress has been made, vigilance and adaptation are paramount as technology evolves and scales. I encourage stakeholders to invest in research and development that prioritizes safety alongside efficiency, ensuring that lithium-ion battery systems contribute positively to our energy future without compromising public well-being. Through shared knowledge and commitment, we can overcome existing hurdles and pave the way for a more secure and sustainable energy ecosystem dominated by advanced lithium-ion battery solutions.
