As a researcher in the field of energy storage, I have witnessed the rapid evolution of all-solid-state batteries, which are poised to revolutionize next-generation energy systems due to their high energy density, enhanced safety, and long cycle life. The core component of these solid-state batteries is the solid-state electrolyte (SSE), which replaces flammable liquid electrolytes, thereby mitigating risks such as thermal runaway and enabling the use of high-capacity electrodes like lithium metal. However, single-component SSEs, including polymers, oxides, and sulfides, face intrinsic limitations that hinder their commercialization. For instance, polymer electrolytes like PEO exhibit poor room-temperature ionic conductivity (often below $10^{-5}$ S/cm), oxide electrolytes such as Li$7$La$_3$Zr$_2$O${12}$ (LLZO) suffer from high interfacial impedance (exceeding 500 Ω/cm$^2$), and sulfide electrolytes like Li$6$PS$_5$Cl, while offering high ionic conductivity (over $10^{-3}$ S/cm), are highly sensitive to moisture and have narrow electrochemical windows. To overcome these challenges, composite solid-state electrolytes have emerged as a promising solution, leveraging synergistic effects between multiple components to achieve balanced performance in ionic conductivity, mechanical stability, and electrochemical compatibility. In this article, I will explore the recent advancements in composite solid-state electrolytes, focusing on filler-type and layered structures, and discuss their implications for the development of high-performance solid-state batteries.
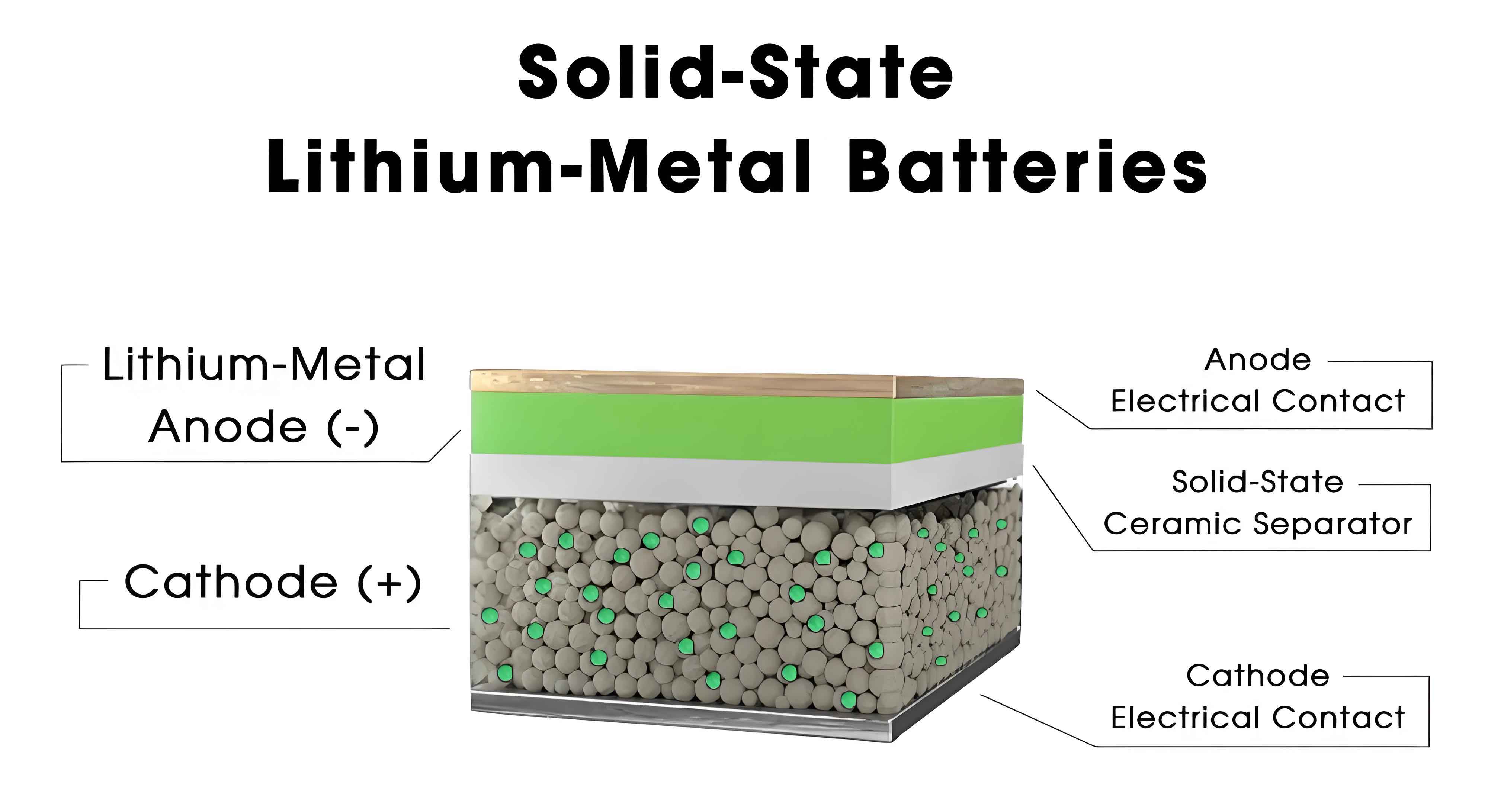
The design of composite solid-state electrolytes is guided by key principles, including chemical compatibility between components to prevent adverse reactions, continuous ion transport pathways to facilitate lithium-ion migration, and balanced mechanical properties to suppress dendrite growth and accommodate volume changes during cycling. Based on structural configurations, composite electrolytes can be broadly classified into filler-type and layered structures. Filler-type composites involve the dispersion of one SSE within another matrix, such as inorganic particles in a polymer base, while layered structures consist of stacked heterostructures with distinct functional layers. Both approaches aim to address the shortcomings of single-component systems and have shown remarkable progress in enhancing the overall performance of solid-state batteries. For example, the ionic conductivity in composite electrolytes can be described by the percolation theory, where the effective conductivity $\sigma{\text{eff}}$ follows a power-law relationship: $$\sigma_{\text{eff}} = \sigma_0 (p – p_c)^t,$$ where $p$ is the volume fraction of the conductive filler, $p_c$ is the percolation threshold, and $t$ is a critical exponent. This equation highlights how filler concentration influences ion transport, and optimizing these parameters is crucial for designing high-performance composites.
In filler-type composites, the incorporation of multidimensional fillers, such as 0D nanoparticles, 1D nanowires, 2D nanosheets, and 3D networks, has been extensively studied to enhance ionic conductivity and mechanical integrity. For instance, 0D nanoparticles like LLZTO in PEO-based electrolytes can reduce polymer crystallinity and provide additional ion transport pathways, leading to ionic conductivities up to $2.1 \times 10^{-4}$ S/cm at room temperature. The mechanism involves Lewis acid-base interactions at the interface, which promote lithium salt dissociation and lower the activation energy for ion migration. Similarly, 1D nanowires, such as Li${0.33}$La${0.557}$TiO$_3$ (LLTO) in PAN matrices, form continuous conduction networks, boosting conductivity to $2.4 \times 10^{-4}$ S/cm. The enhanced performance can be modeled using the effective medium theory, where the composite conductivity $\sigma_c$ is given by: $$\sigma_c = \sigma_m \frac{1 + 2\phi f}{1 – \phi f},$$ with $f = (\sigma_f – \sigma_m)/(\sigma_f + 2\sigma_m)$, where $\sigma_m$ and $\sigma_f$ are the conductivities of the matrix and filler, respectively, and $\phi$ is the filler volume fraction. This equation underscores the importance of filler geometry and dispersion in optimizing ion transport.
Layered composite electrolytes, on the other hand, employ heterostructures to address interface-related issues, such as high impedance and dendrite penetration. For example, sandwich structures like PEO/LATP/PEO have been developed, where the outer PEO layers improve contact with electrodes, and the inner LATP layer provides high ionic conductivity and mechanical strength. This design reduces interfacial impedance from 60,000 Ω to 270 Ω and achieves an ionic conductivity of $4 \times 10^{-5}$ S/cm. Asymmetric layered electrolytes, such as those combining rigid LLZO with flexible polymers, further enhance stability by inhibiting dendrite growth and enabling stable cycling over 3,200 hours. The performance of these layered systems can be analyzed using interfacial resistance models, where the total resistance $R_{\text{total}}$ is the sum of bulk and interfacial contributions: $$R_{\text{total}} = R_b + R_i,$$ and minimizing $R_i$ through layer engineering is critical for practical applications in solid-state batteries.
To provide a comprehensive overview, I have summarized the key properties of single-component SSEs and their roles in composites in Table 1. This table highlights the advantages and limitations of each category, emphasizing how composite strategies leverage these characteristics to achieve synergistic effects.
| Category | Representative Materials | Advantages | Disadvantages | Role in Composite Electrolytes |
|---|---|---|---|---|
| Polymer | PEO, PVDF, PAN | Flexibility, easy processing, low interfacial impedance | Low room-temperature conductivity, poor thermal stability | Flexible matrix for dispersion, dendrite suppression |
| Oxide | LLZO, LATP, LZSP | High chemical stability, high mechanical strength | High interfacial impedance, high sintering temperature | Rigid support, wide electrochemical window |
| Sulfide | Li$_3$PS$_4$, Li$_6$PS$_5$Cl, LGPS | High ionic conductivity, good interfacial contact | Air sensitivity, narrow electrochemical window | Fast ion transport, soft interface contact |
| Halide | Li$_3$YCl$_6$, LiAlCl$_4$ | Wide electrochemical window, solution processability | Air/water sensitivity, low mechanical strength | Interface modification layer |
In polymer/inorganic composite electrolytes, the ion transport mechanisms involve a combination of percolation effects, interfacial interactions, and multidimensional pathways. For example, the addition of 15–30 wt% inorganic fillers can achieve percolation thresholds, leading to conductivities above $10^{-4}$ S/cm. However, excessive filler content may cause aggregation, degrading performance. The interfacial interactions between fillers and polymers can be quantified using the Lewis acid-base concept, where the strength of interaction influences lithium-ion mobility. Additionally, entropy effects play a role, as increased disorder in composite systems reduces crystallinity and activation energy for ion migration. The overall conductivity $\sigma$ can be expressed as: $$\sigma = n e \mu,$$ where $n$ is the charge carrier concentration, $e$ is the elementary charge, and $\mu$ is the mobility, which is enhanced in composites due to optimized pathways.
For inorganic/inorganic composites, such as sulfide-oxide or halide-sulfide combinations, the ion conduction mechanisms include interface-assisted transport, interpenetrating networks, and space-charge layer effects. For instance, composites like LLZO/Li$_6$PS$_5$Cl with a 4:6 mass ratio exhibit ionic conductivities up to $1.27 \times 10^{-3}$ S/cm, owing to the formation of continuous 3D ion channels. The space-charge model describes how interfacial regions facilitate ion migration: $$\Delta \phi = \frac{kT}{e} \ln \left( \frac{\sigma_1}{\sigma_2} \right),$$ where $\Delta \phi$ is the potential difference at the interface, and $\sigma_1$ and $\sigma_2$ are the conductivities of the adjacent phases. This model explains the enhanced conductivity in heterostructured composites used in solid-state batteries.
To illustrate the performance of filler-type inorganic/inorganic composites, Table 2 provides examples of mixing ratios and resulting ionic conductivities. These data demonstrate how strategic compounding can overcome the limitations of individual components, paving the way for advanced solid-state batteries.
| Single-Component SSE | Ionic Conductivity (S/cm) | Composite Sample and Ratio | Mixing Method | Composite Ionic Conductivity (S/cm) |
|---|---|---|---|---|
| LLZO | $1.6 \times 10^{-4}$ | LLZO-LATP (50:50) | Ball milling | $1.3 \times 10^{-6}$ |
| Li$_3$PS$_4$ | $4.0 \times 10^{-4}$ | LLZO/LPS (40:60) | Mixing | $5.36 \times 10^{-4}$ |
| Li$_6$PS$_5$Cl | $2.92 \times 10^{-3}$ | LLZO-LPSC (40:60) | Ball milling | $1.27 \times 10^{-3}$ |
| Li$_3$InCl$_6$ | N/A | LATP/Li$_3$InCl$_6$ (80:20) | Grinding | $1.4 \times 10^{-4}$ |
In layered composite electrolytes, the focus is on optimizing interface stability and suppressing dendrite growth. For example, sulfide-halide heterostructures, such as Li$_3$InCl$_6$/Li$_6$PS$_5$Cl, leverage the high ion migration number of halides for uniform Li$^+$ distribution and the high shear modulus of sulfides for dendrite inhibition. These systems enable all-solid-state batteries to retain over 80% capacity after 3,000 cycles. The interfacial stability can be modeled using the Gibbs free energy of reaction: $$\Delta G = -nFE,$$ where $n$ is the number of electrons, $F$ is Faraday’s constant, and $E$ is the cell potential, which helps predict side reactions and guide material selection for stable interfaces in solid-state batteries.
Beyond filler and layered structures, core-shell configurations represent an innovative approach to composite design. For instance, oxide-coated sulfide particles, such as LLZO@Li${10}$GeP$_2$S${12}$, improve air stability and reduce interfacial resistance, leading to ionic conductivities of $5.8 \times 10^{-3}$ S/cm and extended cycle life. The core-shell structure can be described using a coaxial model, where the effective conductivity $\sigma_{\text{eff}}$ depends on the shell thickness and conductivity: $$\sigma_{\text{eff}} = \sigma_s \frac{2(1-\nu) + (1+2\nu)\kappa}{2(1-\nu) + (1+2\nu)/\kappa},$$ with $\kappa = \sigma_c/\sigma_s$, where $\sigma_c$ and $\sigma_s$ are the core and shell conductivities, and $\nu$ is the volume fraction of the core. This equation highlights the importance of shell properties in enhancing overall performance for solid-state batteries.
The integration of composite electrolytes with electrode materials is crucial for maximizing the performance of solid-state batteries. Filler-type composites, with their homogeneous structures, are well-suited for high-nickel cathodes (e.g., NCM811) and lithium metal anodes, as the continuous filler network provides high ionic conductivity and accommodates volume changes. Layered composites, with their functional zoning, excel in high-voltage systems (e.g., LiCoO$_2$) and silicon-carbon anodes, where the distinct layers optimize interface contact and lithium deposition uniformity. Future research should explore core-shell structures to further enhance compatibility with diverse electrode systems in solid-state batteries.
In conclusion, composite solid-state electrolytes offer a transformative pathway for advancing all-solid-state batteries by addressing the limitations of single-component systems. Filler-type and layered structures provide complementary benefits, with fillers enhancing ion transport and mechanical properties through percolation and interface effects, and layered systems improving interfacial stability and dendrite resistance. However, challenges remain in scaling up these materials, such as achieving thin yet robust electrolytes, ensuring wide temperature adaptability, and maintaining long-term interface stability. Future directions include developing novel materials with self-healing capabilities, employing machine learning for interface design, and advancing low-temperature fabrication techniques like cold sintering. By leveraging multi-scale simulations and high-throughput experiments, we can accelerate the transition of composite electrolytes from lab-scale prototypes to industrial applications, ultimately enabling safer and more efficient solid-state batteries for a sustainable energy future.
The evolution of composite solid-state electrolytes is underpinned by fundamental principles of materials science and electrochemistry. For instance, the ionic conductivity in composites often follows the Arrhenius equation: $$\sigma = A \exp\left(-\frac{E_a}{kT}\right),$$ where $A$ is the pre-exponential factor, $E_a$ is the activation energy, $k$ is Boltzmann’s constant, and $T$ is the temperature. In composites, the activation energy is reduced due to interface effects, leading to higher conductivities at lower temperatures. Additionally, the mechanical properties can be described using models like the rule of mixtures for Young’s modulus: $$E_c = E_m V_m + E_f V_f,$$ where $E_m$ and $E_f$ are the moduli of the matrix and filler, and $V_m$ and $V_f$ are their volume fractions. This equation illustrates how composites achieve balanced mechanical strength for dendrite suppression in solid-state batteries.
As we continue to innovate, the synergy between experimental advancements and theoretical insights will be key to unlocking the full potential of composite solid-state electrolytes. With ongoing research, I am confident that solid-state batteries will soon overcome current barriers, offering unprecedented energy storage solutions for applications ranging from electric vehicles to grid storage. The journey toward commercialization requires collaborative efforts across disciplines, and I am excited to contribute to this transformative field.
