Solid-state batteries represent a transformative advancement in energy storage technology, offering enhanced safety and higher energy density compared to conventional lithium-ion batteries with liquid electrolytes. The replacement of flammable organic electrolytes with solid-state electrolytes (SSEs) mitigates risks such as thermal runaway, leakage, and dendrite formation, while enabling the use of high-capacity electrodes like lithium metal anodes. Among various cathode materials, Li-rich Mn-based layered oxides (LRMOs) have emerged as promising candidates due to their exceptional specific capacity (>250 mAh g−1) and energy density (>1000 Wh kg−1), originating from both cationic (transition metal) and anionic (oxygen) redox reactions. The integration of LRMO cathodes in solid-state batteries holds the potential to achieve energy densities exceeding 600 Wh kg−1, addressing the growing demand for advanced energy storage solutions in electric vehicles and grid storage. However, challenges such as low electronic conductivity, irreversible oxygen release, and unstable electrode-electrolyte interfaces hinder their practical application. This article comprehensively reviews the structural characteristics, electrochemical properties, and recent progress in modifying LRMO cathodes for solid-state batteries, with a focus on strategies to enhance ionic/electronic transport, stabilize interfaces, and improve anionic redox reversibility.
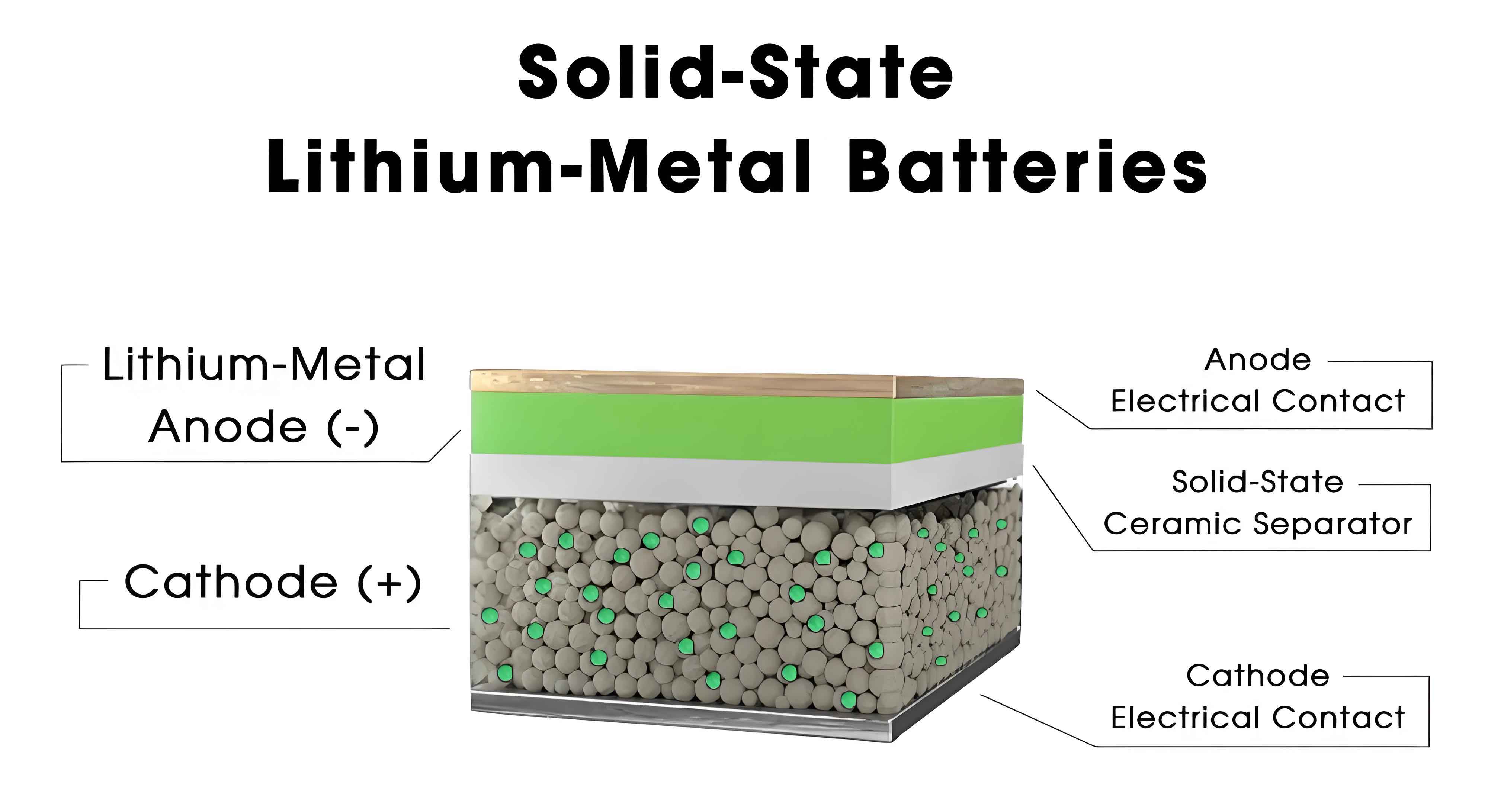
The fundamental architecture of a solid-state battery comprises a solid electrolyte sandwiched between a cathode and an anode, facilitating ion transport while preventing short circuits and degradation. Solid-state batteries leverage inorganic SSEs (e.g., sulfides, oxides, halides) or polymer electrolytes to achieve high mechanical strength and thermal stability. For instance, the ionic conductivity of SSEs can be described by the Arrhenius equation: $$\sigma = A \exp\left(-\frac{E_a}{kT}\right)$$ where $\sigma$ is the ionic conductivity, $A$ is the pre-exponential factor, $E_a$ is the activation energy, $k$ is Boltzmann’s constant, and $T$ is the temperature. This equation highlights the temperature dependence of ion transport in solid-state batteries, which is critical for operational efficiency. The high voltage stability of SSEs is paramount when paired with LRMO cathodes, as these materials often operate above 4.5 V versus Li/Li+, leading to oxidative decomposition at the interface. Strategies to mitigate this include the design of composite cathodes with integrated ion-conducting networks, which enhance the active material utilization and cycle life of solid-state batteries.
LRMO cathodes are typically represented by the general formula $x\text{Li}_2\text{MnO}_3\cdot(1-x)\text{LiMO}_2$ (0 < x < 1, M = Mn, Ni, Co), forming a solid solution between the Li2MnO3 and LiMO2 components. The crystal structure adopts a layered α-NaFeO2-type arrangement, where oxygen atoms form a cubic close-packed lattice, and lithium and transition metal ions occupy octahedral sites. A key feature of the Li2MnO3 component is the presence of Li atoms in the transition metal layers, creating a honeycomb-like ordering of Li-O-Li configurations. These configurations give rise to non-bonding O 2p states, enabling reversible oxygen redox activity, which contributes significantly to the high capacity. The electrochemical performance of LRMO cathodes involves two distinct regions during the initial charge: a sloping region below 4.45 V, associated with lithium extraction and transition metal oxidation, and a plateau above 4.45 V, where Li2MnO3 activation occurs alongside oxygen oxidation. This process can be represented by the reaction: $$\text{Li}_2\text{MnO}_3 \rightarrow \text{MnO}_2 + \text{Li}_2\text{O} + \frac{1}{2}\text{O}_2$$ The irreversible loss of Li2O and oxygen leads to low initial Coulombic efficiency (typically 70–80%) and capacity fading in liquid electrolytes. However, in solid-state batteries, the immobilization of transition metals and suppressed oxygen dissolution can enhance structural integrity, making LRMO cathodes particularly attractive for high-energy solid-state batteries.
Despite their advantages, LRMO cathodes face several challenges in solid-state batteries. The biphasic nature of LRMOs results in incompatibility with certain SSEs, especially at high voltages, leading to interfacial degradation and increased impedance. Additionally, the low electronic conductivity of LRMOs (e.g., ~10−6 S cm−1) necessitates the incorporation of conductive additives, while the sluggish Li+ diffusion kinetics (diffusion coefficient ~10−12–10−14 cm2 s−1) limit rate capability. Oxygen release during cycling promotes the formation of oxygen vacancies, triggering phase transitions from layered to spinel-like and rock-salt structures, which cause voltage decay and capacity loss. The interfacial instability between LRMO cathodes and SSEs is exacerbated by chemical reactions, forming resistive interphases that impede ion transport. For example, the space charge layer effect at sulfide electrolyte interfaces can be modeled using the Poisson-Boltzmann equation: $$\frac{d^2\phi}{dx^2} = -\frac{\rho}{\varepsilon}$$ where $\phi$ is the electric potential, $\rho$ is the charge density, and $\varepsilon$ is the permittivity. This effect leads to Li+ depletion and increased interfacial resistance, underscoring the need for tailored modifications in solid-state batteries.
To address these issues, various modification strategies have been developed for LRMO cathodes in solid-state batteries. These include bulk doping, surface coating, particle morphology control, and mechanochemical processing. For instance, doping with elements like Ru or W enhances structural stability and suppresses oxygen release by strengthening the M-O bonds, as described by the bond dissociation energy: $$E_{\text{bond}} = \frac{D_e}{2} \left(1 – \frac{r_e}{r}\right)^2$$ where $D_e$ is the equilibrium bond energy, $r_e$ is the equilibrium bond length, and $r$ is the actual bond length. Surface coatings with Li3PO4, Li2WO4, or Li2B4O7 provide physical barriers against SSE decomposition while facilitating Li+ transport. The following table summarizes the electrochemical performance of LRMO cathodes in different solid-state battery systems:
| LRMO Cathode Composition | Solid Electrolyte Type | Initial Discharge Capacity (mAh g−1) / CE | Cycling Performance (Cycle Number / Capacity Retention) | Temperature (°C) |
|---|---|---|---|---|
| Li1.2Ni0.13Co0.13Mn0.54O2 | Sulfide | ~225 / ~70% | 1000 cycles / 83% | 27 |
| Li1.2Ni0.13Co0.13Mn0.54O2 | Halide | 248 / 94% | 300 cycles / 81.2% | 25 |
| Li1.2Ni0.13Co0.13Mn0.54O2 | Polymer | ~245 / 84% | 200 cycles / 82.7% | 25 |
| Li1.2Ni0.2Mn0.6O2 | Oxide | 245 / N/A | 200 cycles / 100% | 25 |
The table illustrates that halide-based solid-state batteries achieve higher initial Coulombic efficiency due to better interfacial compatibility, while sulfide systems demonstrate long cycle life. Polymer-based solid-state batteries offer flexibility but require improved ionic conductivity at room temperature. The capacity retention in oxide-based systems is exceptional, attributed to the mechanical stability of the electrolyte-cathode interface.
Further advancements involve the use of single-crystal LRMO cathodes, which minimize grain boundaries and reduce crack formation during cycling. The Li+ diffusion in single crystals follows the Nernst-Einstein relation: $$D = \frac{\sigma kT}{nq^2}$$ where $D$ is the diffusion coefficient, $n$ is the carrier concentration, and $q$ is the charge. Single-crystal particles with sub-micrometer sizes exhibit enhanced rate capability and cycling stability in solid-state batteries. For example, a single-crystal Li1.2Ni0.2Mn0.6O2 cathode delivered a capacity of 316 mAh g−1 at 60°C with 86% retention after 300 cycles, outperforming polycrystalline counterparts. Mechanochemical methods, such as high-energy ball milling, enable the in-situ formation of ion-conducting networks on LRMO surfaces, improving the Li+ transport and oxygen redox reversibility. The integration of carbon nanotubes or graphene as conductive additives further enhances the electronic percolation in composite cathodes, critical for high-performance solid-state batteries.
In conclusion, LRMO cathodes are pivotal for developing next-generation solid-state batteries with high energy density and safety. The synergistic combination of bulk doping, surface engineering, and particle design addresses the challenges of interfacial instability and sluggish kinetics. Future research should focus on optimizing single-crystal synthesis, exploring zero-strain structures to minimize volume changes, and leveraging machine learning for materials discovery. The development of high-voltage-tolerant SSEs and scalable manufacturing processes, such as dry electrode technology, will accelerate the commercialization of LRMO-based solid-state batteries. With continued innovation, these systems are poised to revolutionize energy storage, enabling applications in electric aviation and renewable grid storage.
The potential of solid-state batteries extends beyond conventional applications, offering opportunities in emerging fields like low-altitude economy and portable electronics. The unique properties of LRMO cathodes, such as high capacity and low cost, make them ideal for sustainable energy solutions. However, fundamental studies on the degradation mechanisms and interface dynamics are essential to unlock their full potential. Collaborative efforts between academia and industry will drive the transition from lab-scale breakthroughs to commercial solid-state batteries, ultimately contributing to a carbon-neutral future.
