In my investigation of advanced energy storage systems, I have focused on improving the performance of lithium-ion batteries, particularly under high-voltage conditions. The lithium-ion battery is a cornerstone of modern portable electronics and electric vehicles, but its energy density is often limited by the electrochemical stability of components. Among cathode materials, lithium cobalt oxide (LCO) offers high theoretical capacity and stable cycling, yet raising the charge cutoff voltage to boost energy density induces severe degradation, such as electrolyte oxidation and cobalt dissolution. To address this, I explored the use of bifunctional electrolyte additives, specifically cyanomethyl p-toluenesulfonate (CMPTS), which can simultaneously modify both electrode interfaces. This article details my findings on how CMPTS enhances the electrochemical performance of high-voltage LCO-based lithium-ion batteries, supported by theoretical calculations, experimental data, and mechanistic insights. Throughout this work, the term “lithium-ion battery” will be emphasized to underscore its relevance in energy storage applications.
My study began with density functional theory (DFT) calculations to predict the redox behavior of CMPTS compared to common carbonate solvents like ethylene carbonate (EC), propylene carbonate (PC), and ethyl propionate (EP). The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies were computed to assess oxidation and reduction tendencies. The results indicated that CMPTS has a higher HOMO energy than EC, PC, and EP, suggesting it oxidizes more readily at the cathode. Conversely, its LUMO energy is lower, implying easier reduction at the anode. These properties make CMPTS a promising candidate for prior decomposition to form protective films. The HOMO and LUMO energies can be expressed as:
$$E_{\text{HOMO}}(\text{CMPTS}) > E_{\text{HOMO}}(\text{EC, PC, EP})$$
$$E_{\text{LUMO}}(\text{CMPTS}) < E_{\text{LUMO}}(\text{EC, PC, EP})$$
This theoretical foundation guided my experimental design, where I incorporated CMPTS into a baseline electrolyte of 1 M LiPF6 in EC/PC/EP (3:1:6 by mass) at 1 wt% concentration. The electrochemical performance was evaluated using half-cells (Li/AG and Li/LCO) and full pouch cells (AG/LCO) under high-voltage conditions up to 4.55 V.
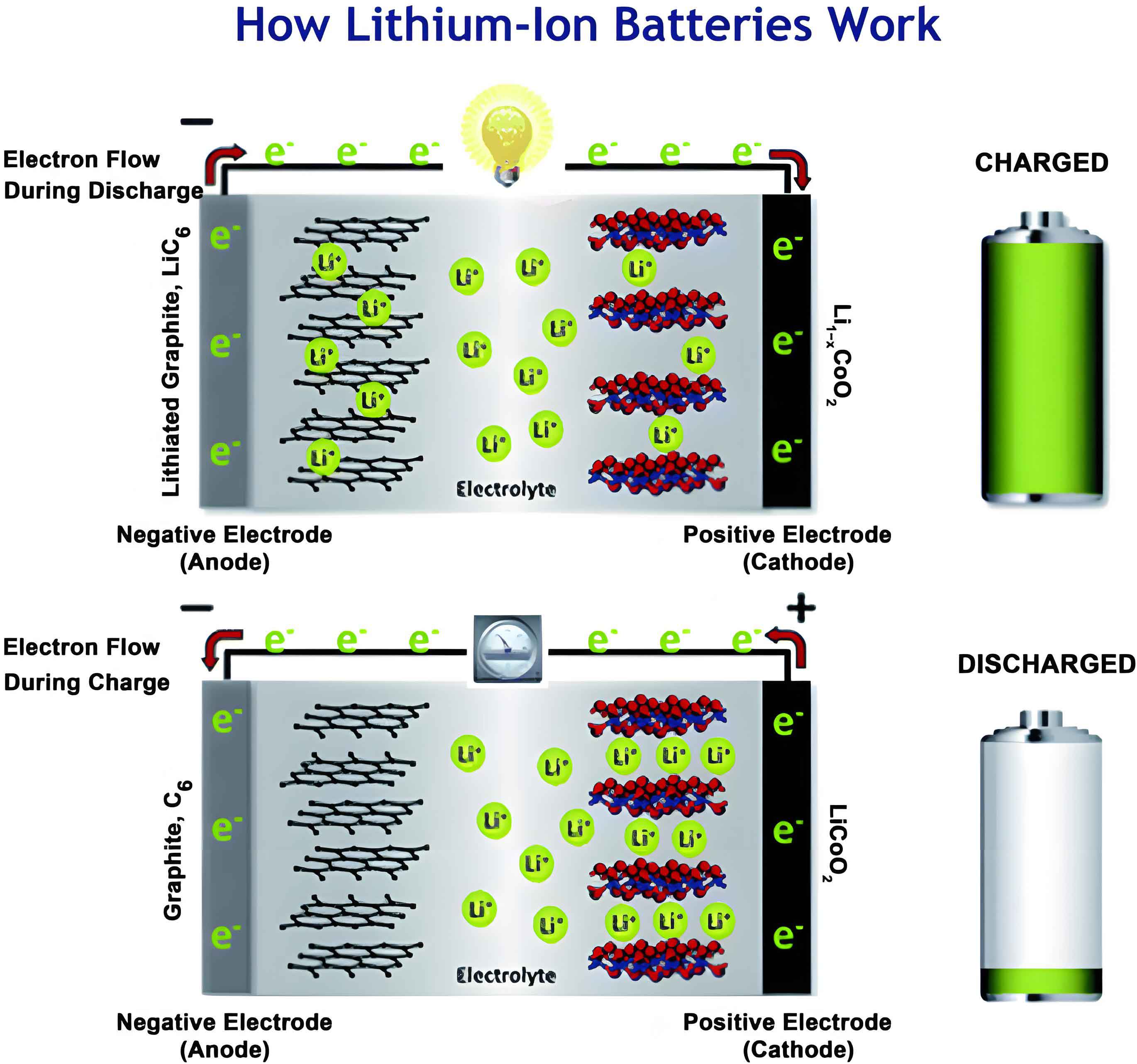
In the anode half-cells, cyclic voltammetry (CV) scans revealed a reduction peak at approximately 1.47 V (vs. Li+/Li) for CMPTS-based electrolytes, absent in the baseline. This peak, observed only in the first cycle, confirmed that CMPTS reduces preferentially on the graphite surface to form a solid electrolyte interphase (SEI). The differential capacity (dQ/dV) analysis corroborated this, showing a distinct reduction signature. Long-term cycling at 0.3 C over 250 cycles demonstrated superior capacity retention with CMPTS (92.8%) compared to the baseline (29.1%). Electrochemical impedance spectroscopy (EIS) fitted data indicated lower SEI resistance for CMPTS-modified cells, as summarized in Table 1. The SEI resistance (RSEI) and charge transfer resistance (Rct) were derived from equivalent circuit modeling, where the total impedance (Z) can be expressed as:
$$Z = R_s + \frac{R_{\text{SEI}}}{1 + j\omega R_{\text{SEI}} C_{\text{SEI}}} + \frac{R_{\text{ct}}}{1 + j\omega R_{\text{ct}} C_{\text{dl}}}$$
Here, Rs is the ohmic resistance, ω is the angular frequency, and C represents capacitance. The lower RSEI values for CMPTS highlight its role in forming a stable SEI, facilitating lithium-ion transport in the lithium-ion battery.
| Electrolyte | Cycle Number | Rs (Ω) | RSEI (Ω) | Rct (Ω) |
|---|---|---|---|---|
| Baseline | 3 | 2.1 | 27.3 | 45.6 |
| Baseline | 250 | 2.5 | 55.1 | 78.9 |
| CMPTS | 3 | 2.0 | 22.5 | 40.2 |
| CMPTS | 250 | 2.3 | 40.0 | 60.4 |
On the cathode side, linear sweep voltammetry (LSV) showed an oxidation current onset at 4.17 V for CMPTS, earlier than solvent oxidation, confirming its preferential oxidation to form a cathode electrolyte interphase (CEI). Leakage current tests under constant voltage at 4.98 V demonstrated lower current for CMPTS cells, indicating suppressed electrolyte decomposition. Cycling performance of Li/LCO half-cells at 0.3 C between 3.0–4.60 V revealed capacity retention of 85.8% with CMPTS versus 62.1% for the baseline after 250 cycles. EIS analysis again showed reduced CEI resistance for CMPTS, as detailed in Table 2. The CEI resistance (RCEI) followed a similar trend, with the overall cell kinetics benefiting from the additive. The rate capability tests further emphasized the advantage, where CMPTS cells maintained higher discharge capacities at increased C-rates, underscoring the improved interface stability in the lithium-ion battery.
| Electrolyte | Cycle Number | Rs (Ω) | RCEI (Ω) | Rct (Ω) |
|---|---|---|---|---|
| Baseline | 3 | 2.3 | 62.2 | 85.7 |
| Baseline | 250 | 2.8 | 108.4 | 120.5 |
| CMPTS | 3 | 2.2 | 35.9 | 58.3 |
| CMPTS | 250 | 2.5 | 45.4 | 75.6 |
To elucidate the interface modifications, I conducted material characterizations. Scanning electron microscopy (SEM) of graphite anodes after 250 cycles showed a cracked SEI for the baseline, whereas CMPTS yielded a dense, uniform film. Transmission electron microscopy (TEM) of LCO cathodes revealed a thick, uneven CEI for the baseline, contrasted with a thin, homogeneous layer for CMPTS. X-ray photoelectron spectroscopy (XPS) analysis provided chemical insights: on the anode, CMPTS-derived SEI contained sulfur species like RSO2Li and Li2SO3 from S 2p spectra, and nitrile groups (–C≡N) from N 1s spectra. On the cathode, CEI exhibited similar sulfur compounds and nitrile signatures, indicating CMPTS incorporation into both interfaces. The nitrile groups are theorized to chelate cobalt ions, inhibiting dissolution and catalytic decomposition. The XPS peak assignments can be summarized with binding energy equations, such as for sulfur species:
$$\text{RSO}_2\text{Li: } BE \approx 170.1 \text{ eV}$$
$$\text{Li}_2\text{SO}_3: BE \approx 168.7 \text{ eV}$$
These findings highlight the bifunctional nature of CMPTS, which enhances the durability of the lithium-ion battery under high-voltage stress.
In full pouch cell evaluations, the impact of CMPTS was pronounced. At room temperature (25°C) with a 0.5 C rate between 3.0–4.55 V, the CMPTS-based lithium-ion battery achieved a capacity retention of 90.4% after 500 cycles, versus 80.8% for the baseline. At elevated temperature (45°C), after 350 cycles, retention rates were 83.2% (CMPTS) and 78.1% (baseline). High-temperature storage tests at 60°C for 7 days showed improved capacity retention and recovery with CMPTS, as quantified in Table 3. The voltage drop during storage was reduced from 2.5% to 0.8%, indicating better interface stability. Cobalt dissolution analysis from stored LCO electrodes revealed lower Co content with CMPTS, confirming its protective role. These results underscore the additive’s efficacy in real-world lithium-ion battery applications.
| Test Condition | Parameter | Baseline | CMPTS |
|---|---|---|---|
| Room Temperature Cycling (500 cycles) | Initial Capacity (mAh/g) | 196.5 | 202.4 |
| Final Capacity (mAh/g) | 158.8 | 182.9 | |
| Capacity Retention (%) | 80.8 | 90.4 | |
| High-Temperature Cycling (45°C, 350 cycles) | Initial Capacity (mAh/g) | 202.1 | 209.3 |
| Final Capacity (mAh/g) | 158.1 | 174.2 | |
| Capacity Retention (%) | 78.1 | 83.2 | |
| Storage at 60°C (7 days) | Capacity Retention (%) | 90.4 | 94.6 |
| Capacity Recovery (%) | 94.7 | 98.6 | |
| Voltage Drop (%) | 2.5 | 0.8 |
The reaction mechanism of CMPTS can be inferred from its dual functional groups. At the cathode, upon delithiation, CMPTS oxidizes, likely through cleavage of S–O or C–O bonds, generating radicals that polymerize or react with electrolyte components to form a sulfur-rich CEI. Simultaneously, the nitrile groups coordinate with cobalt ions, reducing dissolution. At the anode, during lithiation, CMPTS reduces to form species like ROSO2Li, contributing to a robust SEI. This bifunctional action stabilizes both electrodes, as summarized by the following conceptual equations:
Cathode oxidation: $$\text{CMPTS} \rightarrow \text{RSO}_2^\bullet + \text{other radicals} \rightarrow \text{CEI (with Li}_2\text{SO}_3, \text{RSO}_2\text{Li)}$$
Anode reduction: $$\text{CMPTS} + e^- \rightarrow \text{R–OSO}_2^- + \text{Li}^+ \rightarrow \text{SEI (with Li}_2\text{SO}_4, \text{ROSO}_2\text{Li)}$$
The nitrile coordination: $$\text{–C≡N} + \text{Co}^{n+} \rightarrow \text{complex, inhibiting Co dissolution}$$
This mechanism explains the enhanced performance, as the additive preferentially forms films that minimize parasitic reactions, a key advancement for high-voltage lithium-ion battery technology.
Differential scanning calorimetry (DSC) of cycled electrodes further supported the thermal stability of CMPTS-derived interfaces. Baseline electrodes showed exothermic peaks near 400°C, indicative of film decomposition, while CMPTS electrodes exhibited smoother curves, suggesting a more thermally stable SEI/CEI. This aligns with the improved high-temperature performance, critical for safety in lithium-ion battery systems. The thermal behavior can be modeled with Arrhenius-type equations for decomposition kinetics:
$$\frac{d\alpha}{dt} = A e^{-E_a/RT} (1-\alpha)^n$$
where α is the conversion degree, A is the pre-exponential factor, Ea is activation energy, R is the gas constant, T is temperature, and n is the reaction order. The higher Ea for CMPTS interfaces would correlate with better stability.
In summary, my research demonstrates that the bifunctional additive CMPTS significantly boosts the performance of high-voltage LCO-based lithium-ion batteries. By participating in SEI and CEI formation, it reduces interface resistance, suppresses electrolyte oxidation and cobalt dissolution, and enhances thermal stability. The theoretical and experimental evidence, including DFT calculations, electrochemical tests, and material characterizations, validates its dual role. This approach offers a simple yet effective strategy for designing next-generation electrolytes, paving the way for safer and more durable lithium-ion batteries operating at elevated voltages. Future work could explore variations of CMPTS or combinations with other additives to further optimize the lithium-ion battery lifecycle. The continuous innovation in electrolyte additives remains vital for advancing energy storage solutions, and my findings contribute to this ongoing effort in lithium-ion battery research.
