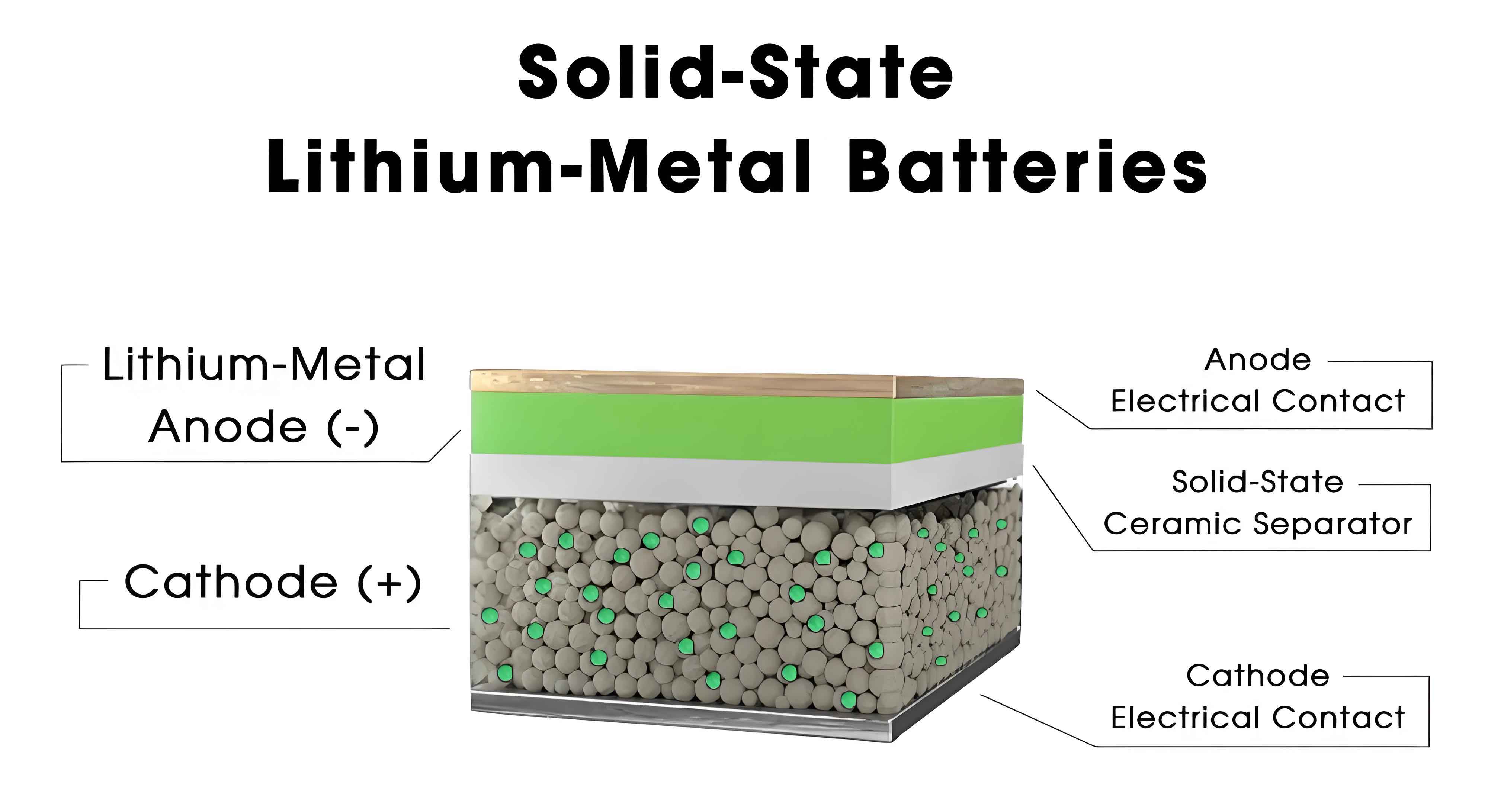
In the pursuit of next-generation energy storage systems, all-solid-state batteries have emerged as a highly promising technology due to their potential for enhanced safety, higher energy density, and longer cycle life compared to conventional liquid electrolyte batteries. Among various solid electrolyte materials, sulfide-based solid electrolytes stand out for their high ionic conductivity and favorable mechanical properties. However, the fabrication of thin, flexible electrolyte layers remains a critical challenge for scaling up production and improving performance. Traditional wet processing methods often involve organic solvents, which can lead to environmental concerns, high costs, and issues like crack formation in electrodes. In contrast, dry fabrication techniques offer a solvent-free alternative that is more eco-friendly and economically viable, yet they face limitations such as poor adhesion and mechanical instability when using binders like polytetrafluoroethylene (PTFE).
We have developed an innovative dry fabrication approach based on fusion bonding technology to produce ultra-thin sulfide solid electrolyte membranes with exceptional flexibility and performance. This method addresses key drawbacks of existing dry processes by enabling strong interfacial adhesion, high mechanical strength, and efficient stress dissipation, which are crucial for preventing mechanical failures in all-solid-state batteries. Our research focuses on optimizing this technique for high-area-loading electrodes, such as LiNi0.83Co0.11Mn0.06O2 (NCM83), and integrating them with porous aluminum current collectors to achieve seamless interfaces and long-term stability. This advancement not only enhances the practicality of sulfide-based all-solid-state batteries but also paves the way for large-scale manufacturing.
The core of our work lies in the fusion bonding process, which involves melting a binder material to create a cohesive electrolyte layer without solvents. This results in a uniform, thin membrane that exhibits high ionic conductivity and robust mechanical properties. To quantify the improvements, we have conducted extensive experiments comparing our dry fabrication method with conventional techniques. For instance, the ionic conductivity of our sulfide solid electrolyte membrane can be modeled using the Arrhenius equation, which describes the temperature dependence of ionic transport:
$$ \sigma = A \exp\left(-\frac{E_a}{kT}\right) $$
where (\sigma) is the ionic conductivity, (A) is the pre-exponential factor, (E_a) is the activation energy, (k) is Boltzmann’s constant, and (T) is the temperature. Our measurements show that the fusion-bonded membrane achieves conductivities exceeding 10−3 S/cm at room temperature, which is competitive with liquid electrolytes and essential for high-performance all-solid-state batteries.
In terms of mechanical performance, the stress-strain behavior of the electrolyte membrane is critical for withstanding internal pressures during battery operation. We use a linear elastic model to approximate the mechanical response:
$$ \sigma_m = E \epsilon $$
where (\sigma_m) is the mechanical stress, (E) is the Young’s modulus, and (\epsilon) is the strain. Our fusion-bonded membranes demonstrate a high Young’s modulus and excellent strain tolerance, reducing the risk of fracture. The table below summarizes key properties compared to other fabrication methods, highlighting the advantages of our dry technique for all-solid-state batteries.
| Fabrication Method | Ionic Conductivity (S/cm) | Thickness (μm) | Mechanical Strength (MPa) | Adhesion Quality |
|---|---|---|---|---|
| Wet Processing (Solvent-based) | ~10−3 | 20-50 | 50-100 | Moderate, prone to cracks |
| Dry Processing (PTFE Binder) | ~10−4 | 30-60 | 20-50 | Poor, limited bonding |
| Fusion Bonding (Our Method) | >10−3 | 10-20 | 100-150 | Excellent, seamless interface |
The integration of this electrolyte into all-solid-state batteries involves assembling it with high-capacity electrodes. For the NCM83 cathode, we achieve area loadings of over 50 mg/cm2, which is vital for high energy density. The interfacial resistance between the electrolyte and electrode plays a significant role in overall battery performance. We model this using a simplified equivalent circuit for all-solid-state batteries:
$$ R_{\text{total}} = R_{\text{bulk}} + R_{\text{interface}} $$
where (R_{\text{total}}) is the total resistance, (R_{\text{bulk}}) is the bulk resistance of the electrolyte, and (R_{\text{interface}}) is the interfacial resistance. Our fusion bonding technique minimizes (R_{\text{interface}}) by promoting intimate contact, as evidenced by electrochemical impedance spectroscopy results showing low resistance values.
Cycling performance is another critical metric for all-solid-state batteries. We have tested our batteries over hundreds of cycles, observing minimal capacity fade. The capacity retention can be expressed as a function of cycle number (n):
$$ C(n) = C_0 \exp(-kn) $$
where (C(n)) is the capacity at cycle (n), (C_0) is the initial capacity, and (k) is the degradation rate constant. Our all-solid-state batteries exhibit low (k) values, indicating superior longevity. This is attributed to the stable interface and mechanical integrity provided by the dry-fabricated electrolyte, which prevents dendrite formation and other failure modes common in liquid electrolyte systems.
Furthermore, the economic and environmental benefits of dry fabrication are substantial. By eliminating solvents, we reduce manufacturing costs and waste, making all-solid-state batteries more accessible for applications such as electric vehicles and grid storage. The table below compares the lifecycle environmental impact and cost factors for different battery fabrication methods, underscoring the advantages of our solvent-free approach for sustainable all-solid-state batteries.
| Aspect | Wet Processing | Dry Processing (Traditional) | Fusion Bonding (Our Method) |
|---|---|---|---|
| Solvent Usage | High, hazardous | None | None |
| Energy Consumption | Moderate (drying required) | Low | Low to moderate |
| Production Cost (per unit) | High | Medium | Low |
| Scalability | Limited by solvent handling | Good | Excellent |
In addition to performance metrics, we have explored the fundamental materials science behind the fusion bonding process. The binder material’s properties, such as melting point and viscosity, are optimized to ensure uniform distribution and strong adhesion. We use differential scanning calorimetry (DSC) to characterize the thermal behavior, with the heat flow (\dot{Q}) described by:
$$ \dot{Q} = m C_p \frac{dT}{dt} $$
where (m) is the mass, (C_p) is the specific heat capacity, and (\frac{dT}{dt}) is the heating rate. This analysis confirms that the binder melts uniformly, facilitating the formation of a continuous electrolyte layer in all-solid-state batteries.
The mechanical stress distribution within the battery during cycling is also a key consideration. Finite element analysis (FEA) simulations show that our fusion-bonded electrolyte effectively dissipates stress, reducing the likelihood of delamination. The stress (\sigma) at any point can be modeled using Hooke’s law for anisotropic materials:
$$ \sigma_{ij} = C_{ijkl} \epsilon_{kl} $$
where (C_{ijkl}) is the stiffness tensor and (\epsilon_{kl}) is the strain tensor. Our results indicate that the electrolyte maintains integrity under cyclic loading, which is essential for the durability of all-solid-state batteries.
Looking ahead, we are investigating the integration of this dry fabrication method with other advanced materials, such as high-nickel cathodes and silicon anodes, to further enhance energy density. The development of all-solid-state batteries is a multidisciplinary effort, and our work on solvent-free processes represents a significant step toward commercialization. By addressing interfacial and mechanical challenges, we believe that sulfide-based all-solid-state batteries will play a pivotal role in the future of energy storage.
In conclusion, our fusion bonding technique for dry fabrication of sulfide solid electrolyte membranes offers a breakthrough in the production of all-solid-state batteries. It combines high ionic conductivity, excellent mechanical properties, and environmental benefits, making it a viable solution for large-scale applications. As research progresses, we anticipate further optimizations that will unlock the full potential of all-solid-state batteries, driving innovation in renewable energy and transportation sectors. The repeated emphasis on solid-state battery technologies in this work underscores their importance, and we are committed to advancing this field through continuous improvement and collaboration.
