Solid-state batteries represent a transformative advancement in energy storage technology, leveraging solid electrolytes to address critical limitations of conventional liquid-based systems. The inherent safety risks associated with flammable organic electrolytes in traditional lithium-ion batteries, coupled with energy density constraints imposed by graphite anodes, have propelled the development of solid-state batteries as a promising alternative. By replacing liquid components with solid electrolytes, these systems potentially enable the integration of high-energy-density materials like lithium metal anodes and high-voltage cathodes, while mitigating thermal runaway and leakage issues. However, the transition from solid-liquid to solid-solid interfaces introduces complex challenges that directly impact ion transport kinetics, mechanical stability, and long-term cyclability. This article delves into the fundamental characteristics of solid-solid interfaces, explores their degradation mechanisms, and evaluates cutting-edge characterization techniques essential for optimizing solid-state battery performance.
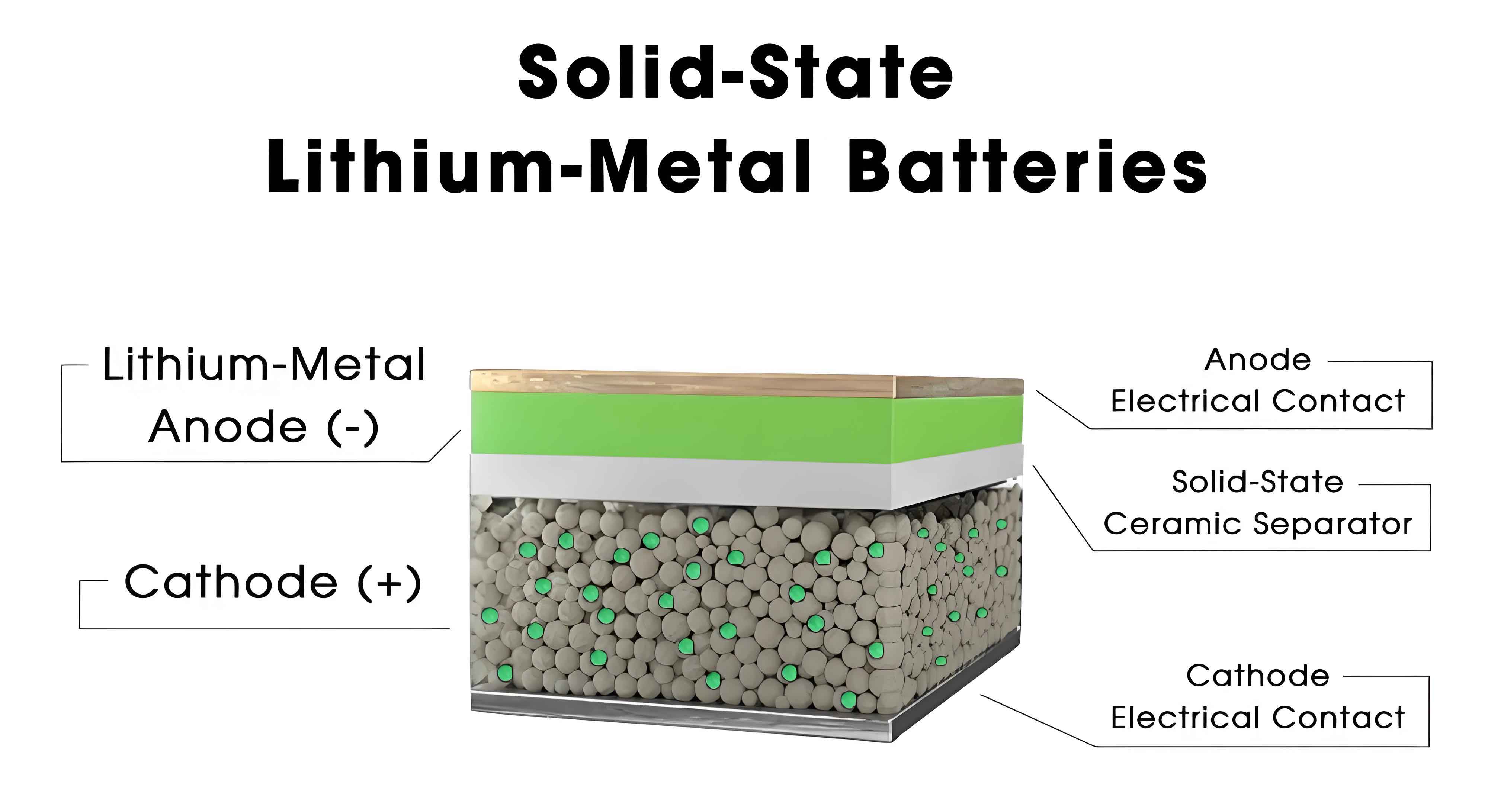
The solid-solid interface in solid-state batteries is a critical determinant of overall cell performance, influencing ionic conductivity, charge transfer efficiency, and structural integrity. Unlike liquid electrolytes that conform to electrode surfaces, solid electrolytes exhibit rigid contact, leading to limited interfacial area and elevated impedance. Key interfacial properties include point contact characteristics, where mechanical stress and material rigidity reduce effective contact area, necessitating external pressure to maintain adhesion. For instance, sulfide-based solid-state batteries often require pressures up to 20 MPa, while polymer-based systems may operate under 2–10 MPa. Ion transport across these interfaces is governed by the electrolyte’s intrinsic conductivity and the electrode’s ionic percolation network. Composite electrodes incorporating ion-conductive coatings, such as LLZO or halide electrolytes, have been developed to facilitate lithium-ion diffusion within the electrode matrix. Furthermore, interfacial reactions—such as oxidation of sulfide electrolytes or reduction at lithium metal interfaces—can form resistive layers, exacerbating capacity fade. Mechanical stresses arising from electrode volume changes during cycling also pose risks of crack propagation and delamination. Understanding these dynamics is crucial for designing robust solid-state batteries.
Degradation at solid-solid interfaces occurs through two primary pathways: contact deterioration and side reactions. Contact deterioration stems from mechanical strain induced by electrode expansion/contraction, leading to crack formation and interfacial detachment. In situ X-ray computed tomography (XCT) has revealed vertical cracking patterns in silicon anodes paired with sulfide electrolytes, where stress accumulation during delithiation initiates fractures that partially heal upon lithiation. Electrode microstructure plays a pivotal role; for example, smaller electrolyte particles enhance composite homogeneity, alleviating stress concentrations. Conversely, side reaction degradation involves chemical processes like lithium dendrite growth, element interdiffusion, and space charge layer formation. Lithium dendrites, driven by localized current densities and electronic conductivity in solid electrolytes, can penetrate electrolytes, causing short circuits. Elemental interdiffusion between electrodes and electrolytes—observed via transmission electron microscopy—forms amorphous interfacial layers that increase impedance. Space charge layers, resulting from lithium chemical potential gradients, create nanoscale regions of ion depletion/accumulation, impeding ion transport. Techniques like electron holography have visualized potential gradients exceeding 100 mV/nm at oxide cathode–sulfide electrolyte interfaces, highlighting the need for interfacial engineering.
Advanced characterization techniques are indispensable for probing solid-solid interfaces in solid-state batteries. Electrochemical methods, such as electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV), provide insights into interfacial kinetics and reaction reversibility. EIS data, when analyzed using distribution of relaxation times (DRT), can decouple contributions from multiple interfaces, revealing resistance evolution under operating conditions. For instance, DRT analysis of sulfide-based solid-state batteries has identified charge transfer resistances dominating at high frequencies, while low-frequency responses correlate with diffusion limitations. The impedance of a typical solid-state battery cell can be modeled using the equation:
$$Z(\omega) = R_{\text{bulk}} + \frac{R_{\text{ct}}}{1 + (j\omega R_{\text{ct}} C_{\text{dl}})^\alpha} + \frac{\sigma_{\text{W}}}{\sqrt{j\omega}}$$
where (R_{\text{bulk}}) represents bulk electrolyte resistance, (R_{\text{ct}}) denotes charge transfer resistance, (C_{\text{dl}}) is double-layer capacitance, (\alpha) signifies dispersion factors, and (\sigma_{\text{W}}) corresponds to Warburg diffusion coefficients. CV studies further elucidate interfacial stability by detecting oxidation/reduction peaks associated with electrolyte decomposition or phase transitions.
Non-destructive techniques like XCT and ultrasonic scanning enable real-time monitoring of interfacial morphology and mechanical integrity. XCT imaging has captured dendrite propagation and crack dynamics in solid-state batteries under varying current rates, revealing that high-rate charging accelerates defect formation. Ultrasonic methods detect stress distribution and gas accumulation at interfaces, with studies showing that cyclic volume changes lead to localized detachment and increased impedance. These approaches are complemented by post-mortem analyses, including scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDS), which visualize elemental diffusion and crack networks. Atomic force microscopy (AFM) measures nanoscale mechanical properties, such as adhesion strength and elastic modulus, providing correlations between interfacial stress and degradation. For chemical composition analysis, X-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (TOF-SIMS) identify reaction products like Li₂S or Li₃P at degraded interfaces. Transmission electron microscopy (TEM) techniques, including electron energy loss spectroscopy (EELS), directly resolve space charge layers by mapping lithium ion distributions across interfaces.
Emerging online sensing technologies offer dynamic interfacial monitoring. Optical coherence tomography (OCT) tracks dendrite growth in operando, with resolution comparable to SEM. Fiber Bragg grating (FBG) sensors embedded in cells measure strain variations during cycling, correlating mechanical stress with capacity fade. These innovations pave the way for closed-loop control systems in solid-state batteries.
Future strategies for interfacial optimization focus on composite electrolytes, functional additives, and advanced manufacturing. Composite electrolytes blending polymers with inorganic fillers (e.g., LLZO or Li₃PS₄) improve mechanical compliance and ionic conductivity. Additives like trimethylsilyl compounds form stable cathode electrolyte interphase (CEI) layers, suppressing sulfide oxidation. Dual-additive systems, such as LiBODFP and LiDFOB in PEO-based electrolytes, enhance interfacial stability on both electrodes. Manufacturing processes like warm pressing or sol-gel deposition reduce interfacial voids, mitigating impedance rise. The following table summarizes key interfacial modification approaches:
| Strategy | Mechanism | Impact on Solid-State Batteries |
|---|---|---|
| Composite Electrolytes | Combines polymer flexibility with inorganic conductivity | Reduces interfacial resistance; enhances mechanical stability |
| Functional Additives | Forms protective CEI/SEI layers | Suppresses side reactions; extends cycle life |
| Surface Coating | Prevents element interdiffusion | Maintains interfacial integrity; improves rate capability |
| Process Optimization | Minimizes voids via controlled pressure/temperature | Enhures uniform contact; reduces dendrite nucleation |
Mathematical modeling further aids in interfacial design. The ion flux across a solid-solid interface can be described by the Nernst-Planck equation:
$$J_i = -D_i \nabla c_i – \frac{z_i F}{RT} D_i c_i \nabla \phi + c_i v$$
where (J_i) is the flux of species (i), (D_i) is the diffusion coefficient, (c_i) is concentration, (z_i) is charge number, (F) is Faraday’s constant, (R) is the gas constant, (T) is temperature, (\phi) is electrostatic potential, and (v) is velocity. Incorporating stress effects, the interfacial overpotential (\eta) relates to mechanical strain (\epsilon) via:
$$\eta = \eta_0 + \kappa \epsilon$$
where (\eta_0) is the strain-free overpotential and (\kappa) is a coupling coefficient. Such models help predict performance under operational stresses.
In conclusion, the evolution of solid-state batteries hinges on resolving interfacial challenges through multidisciplinary approaches. Characterization techniques spanning electrochemistry, microscopy, and spectroscopy provide critical insights into degradation mechanisms, while material innovations and processing advances enable more resilient interfaces. As research progresses, the integration of real-time monitoring and computational modeling will accelerate the development of high-performance solid-state batteries for electric vehicles and grid storage, ultimately fulfilling their promise of enhanced safety and energy density.
| Technique | Resolution | Applications | Limitations |
|---|---|---|---|
| Electrochemical Impedance Spectroscopy (EIS) | Macroscopic | Interface resistance quantification; kinetic analysis | Cannot resolve spatial heterogeneity |
| X-Ray Computed Tomography (XCT) | μm to nm | 3D morphology; crack/dendrite visualization | Limited chemical sensitivity; radiation damage |
| Scanning Electron Microscopy (SEM) | nm | Surface topography; elemental mapping (with EDS) | Vacuum requirements; sample destruction |
| Transmission Electron Microscopy (TEM) | Atomic | Space charge layer imaging; lattice structure | Complex sample preparation; beam sensitivity |
| X-Ray Photoelectron Spectroscopy (XPS) | nm | Chemical composition; oxidation states | Surface-only analysis; ultra-high vacuum needed |
| Ultrasonic Scanning | μm | Stress distribution; defect detection | Low spatial resolution; coupling medium required |
The continuous refinement of these methodologies will deepen our understanding of solid-solid interfaces, enabling the rational design of next-generation solid-state batteries. By addressing interfacial instabilities through material engineering and advanced diagnostics, the path toward commercialization of solid-state batteries becomes increasingly viable, promising a new era of energy storage solutions.
